Introduction
Osteoarthritis (OA) is a persistent degenerative condition characterized by the deterioration of articular cartilage, subchondral bone sclerosis, and the development of osteophytes. It mostly impacts the knees, hips, hands, and spine. The principal clinical manifestations include chronic pain, joint instability, stiffness, and radiological changes. 1
The intra-articular administration of monosodium Iodoacetate (MIA) is regarded as an effective model for knee osteoarthritis (OA). MIA is a straightforward, non-invasive, and rapidly advancing chemically induced animal model of osteoarthritis primarily utilized to assess pain behaviour. MIA promotes chondrocyte apoptosis accompanied with early alterations in functional, radiological, histological, and biochemical parameters resembling human osteoarthritis.2
The predominant pharmacologically active component in the OrthoGo formulation is hemp seed powder, in conjunction with glucosamine sulfate potassium chloride and collagen peptide. Despite Seed's recognized anti-inflammatory capabilities, its application for pain reduction may be hindered by its euphoric effects. In contrast, omega fatty acids and cannabinoids found in hemp seed powder exhibit anti-inflammatory, antinociceptive, and antioxidant properties without the psychotropic effects of hemp, rendering it a promising candidate for the management of pain and inflammatory conditions, including various types of arthritis. Previous studies have shown that hemp may modulate pain in synovial joints. Fundamental scientific study has established that the endocannabinoid system exists in the synovium and can regulate nociception and inflammation in synovial joints in both humans and animals. 3, 4
Due to the numerous limits of pharmacologic therapies for osteoarthritis, researchers have been compelled to seek other medicines to alleviate joint inflammation and pain. This experimental investigation intends to elucidate the effects of the OrthoGo formulation, which contains hemp seed powder, on MIA-induced osteoarthritis.
Materials and Methods
Experimental animals
The Institutional Animal Ethical Committee (IAEC) at K.K. College of Pharmacy in Chennai, India, sanctioned the utilization of animals for the present study (KKCP/2023/05). The animal studies adhered to the rules established by the Committee for Control and Supervision of Experimental Animals (CCSEA). The present study evaluated anti-osteoarthritic activity in healthy albino Sprague Dawley rats aged 8 to 10 weeks and weighing between 150 and 200 grams. The animals were kept in a room with regulated temperature (22 ± 2°C) and humidity (60%-80%), and subjected to light-dark cycles of 12 hours. All experimental techniques were conducted during the light phase. The animals were provided with food and water ad libitum.
Treatment protocol
The rats were divided into groups as follows: a saline group (n =6) received 50 µL of sterile saline 0.9%; an MIA 0.5 mg group (n = 6) received 0.5 mg of MIA; a standard group (n = 6) received Diclofenac Sodium; a low dose treatment group (n = 6) received OrthoGo granules 100 mg / kg high dose treatment group (n = 6) received OrthoGo granules 400 m/ kg. All dosages of MIA were diluted in 50 µL of sterile saline solution with a concentration of 0.9% (Table 1).
Table 1
Animal grouping and treatment protocol
Induction of OA
The rats were anesthetized, and the limb was bent at a 90° angle following local anesthesia. A syringe was introduced via the intra-patellar ligament to inject the solution into the intraarticular region of the right knee. The control group received an intra-articular injection of sterile saline.
Evaluation of analgesia (Eddy’s Hotplate)
The initial response time of the animals subjected to a continuous temperature of 55°C on the hot plate was assessed by monitoring jumping or paw licking behaviour. Animals generally display a reaction within 6 to 8 seconds. A 15-second cutoff restriction will be implemented to avert any injury to the paws. Subsequent to induction and therapy, the animals' response times on the hot plate apparatus were documented. As the animals' reaction time increases due to pharmacological intervention, 15 seconds will be deemed the maximum duration for analgesia, necessitating their removal from the analgesiometer to avert injury.
Inclined plane
The assessment concentrated on evaluating the long-term grip strength of the forelimbs and hindlimbs in rats on a weekly basis. The inclined plane employed in this study consisted of a 75 cm x 15 cm adjustable acrylic panel positioned at an angle. The experimental approach involved positioning the rats on the plane. The inclined plane was raised to an angle of 45 degrees. The grip strength of the rat's skeletal muscle was evaluated by timing how long it took for the rat to descend to the bottom of the plane.
Stair case
The staircase test assesses a rat's proficiency in utilizing its forelimbs to grasp and retrieve small things. The assessment is often referred to as the "paw-reaching test." The testing apparatus comprises a staircase. In our testing setup, the final step of the staircases is baited with pellets. The test's outcome measure is the duration required for the rat to seize the pellet.
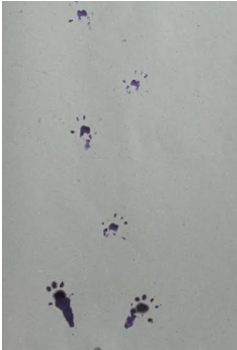
Gait test foot print method
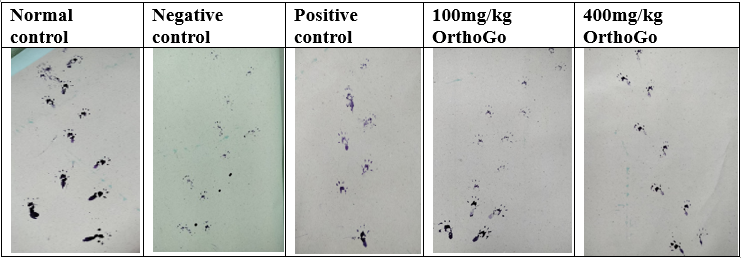
The forelimb paw prints of rats were coated with commercially available black ink, and a rat was positioned on a strip of paper (4.5 cm width, 80 cm long) on a well-lit runway to traverse towards a dark goal box. Paw prints recorded at the start (10 cm) and conclusion (10 cm) of the run were omitted due to variations in velocity. Runs during which the rat exhibited stops or notable decelerations, as noted by the experimenter, were omitted from the analysis. Gait metrics, such as stride length, stride length variability, base of support, cadence, and average speed, were assessed.
Radio graphical examination
On day 28, animals were sedated using ketamine/xylazine (100/10 mg/kg, intraperitoneally). Subsequently, the rat's right knee was flexed in the anterior-posterior position (AP view, angle 10°) and in the lateral position (angle 30°) and the X-ray images were recorded.
Histopathology
The right knees of the rats were surgically excised, labelled, and stored in formaldehyde. The knees were subjected to decalcification in a 5% formic acid solution for up to 96 hours. The knees were subsequently incised along a sagittal plane, bisecting the femur, patella, and tibia. The specimens were subjected to a 6-hour rinse under running water, thereafter processed for histopathological examination, and stained with hematoxylin and eosin. The histological samples were analysed and photographed using a microscope with a digital imaging system.
Statistical analysis
GraphPad Prism software was used to statistically evaluate the results that were produced. The final values were illustrated as Mean ± SD of measurements. The variances between groups were measured using One-Way ANOVA and Dunnett’s multiple comparison with p value <0.05 considered as statistically significant.
Results
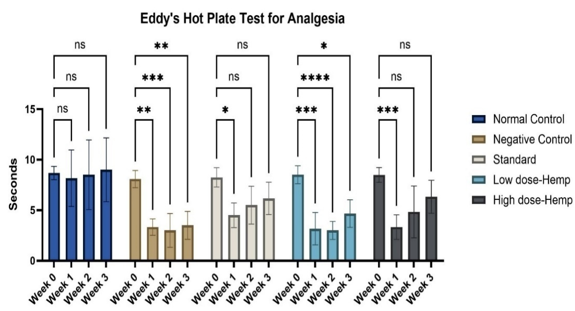
Eddy’s hotplate
The control group often exhibited a higher tolerance for thermal discomfort, whereas rats stimulated with MIA demonstrated less tolerability. In comparison to other groups, the OrthoGo formulation at a dosage of 400 mg/kg demonstrates a progressive enhancement in analgesic efficacy. It yields an equivalent impact to the standard (Figure 5, Figure 6).
Values are expressed as mean ± SD (n=6). Data analysed by one way ANOVA followed by Dunnett's multiple comparisons test. Graphical representation of analgesic activity of the treatment drug using eddy’s hotplate. ns=non-significant, *p<0.05, **p<0.01, ****p<0.001
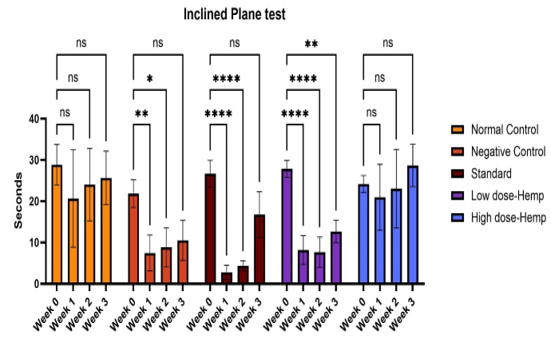
Inclined plane
The time duration required for the control rats to descend the slanted plane is significantly greater than that of the other groups. The rats with a 400 mg/kg dose of OrthoGo formulation exhibited an extended duration to reach the bottom of the incline in the third week compared to the other groups. This indicates that the recovery of muscle strength is superior in the 400 mg/kg OrthoGo dosage group compared to the others.
Values are expressed as mean ± SD (n=6). Data analysed by one way ANOVA followed by Dunnett's multiple comparisons test. Graphical representation of Grip strength of the treatment drug using Inclined plane. ns=non-significant, *p<0.05, **p<0.01, ****p<0.001
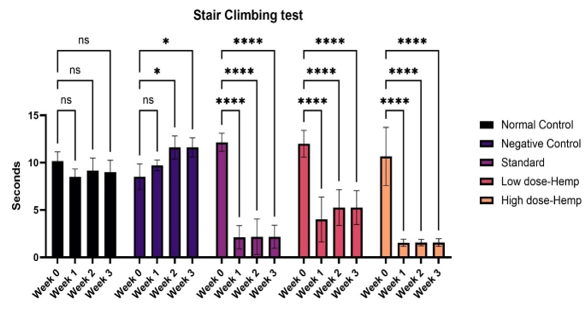
Stair climbing
The number of steps ascended by normal rats exceeded that of the other groups with osteoarthritis. In the negative control group, it exhibited a considerable reduction. The group treated with OrthoGo granules at 400 mg/kg exhibited a significant enhancement comparable to the control group and beyond the usual treatment.
Values are expressed as mean ± SD (n=6). Data analysed by one way ANOVA followed by Dunnett's multiple comparisons test. Graphical representation of Grip strength of the treatment drug using Stair case. ns=non-significant, *p<0.05, **p<0.01, ****p<0.001
Gait test
The number of steps taken by normal rats surpassed those of the other groups with osteoarthritis. The negative control group demonstrated a significant decrease. The group with OrthoGo granules at 400 mg/kg shown a notable improvement, comparable to the control group and beyond standard treatment outcomes.
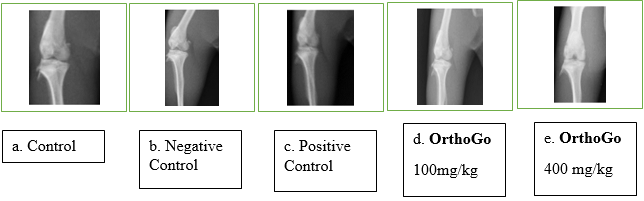
Radiographic examination
The negative control group exhibited progressive radiographic alterations in the knee joint throughout the trial period, while the control group retained a normal look. The negative control group has Kellegren-Lawrence Grade 4, indicating substantial osteophyte growth, significant joint space constriction with pronounced sclerosis, and clear deformity of the bone ends. In the conventional and Ortho Go therapy groups, it was classified as Grade 2, indicating potential constriction of the joint space accompanied by evident osteophyte formation.
X-ray of knee in Normal, Negative Control, Standard, OrthoGo 100 mg / kg and OrthoGo 400 mg/ kg. A. Normal control group shows normal opacity and bicondylar; b Negative control group shows presence of bone loss with wear of the cartilage of the knee joint and joint instability. C. Positive control - opacity essentially concentrated at the femoral condyle corresponding to a scar bone callus associated with lysis of the articular cartilage.
d. OrthoGo 100 mg/ kg shows the presence of an opacity essentially concentrated at the femoral condyle corresponding to a scar bone callus associated with lysis of the articular cartilage. OrthoGo 400 mg/ kg showed the presence of an opacity substantially concentrated at the femoral condyle corresponding to bone callus, associated with lysis of articular cartilage.
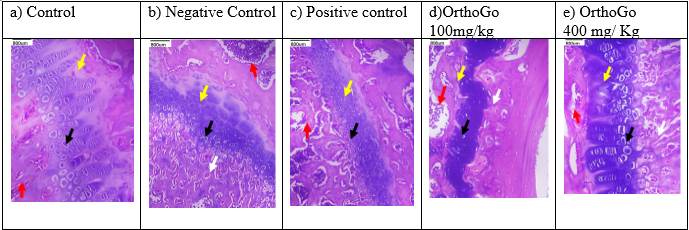
Histopathology
The histological examination indicated degeneration in all induction groups, while synovitis was noted in the negative control group. elevated dosage The Orthogo 400mg/kg treatment safeguarded the knee joints, as seen by the absence of synovitis, demonstrating its anti-osteoarthritic efficacy.
Histopathology of rat knee; Red arrow represents the bone marrow black arrow represents the chondrocytes and white arrow represents the synovitis. a) Control group: normal articular cartilage, normal chondrocytes with Adjacent normal lamellar bone with marrow elements are seen. no synovitis is seen. b) Negative control: mild degeneration of the chondrocytes, synovitis. c) Positive control: normal articular cartilage with normal chondrocytes, no synovitis is seen. d) OrthoGo100mg/kg: showed degenerated irregular articular cartilage with moderate synovitis e) OrthoGo 400 mg/kg showed mild degeneration of the chondrocytes (black arrow) with no synovitis.
Discussion
This study employs animal models for screening analgesic activity, specifically pain-state models utilizing thermal stimuli, including the tail-flick and hot plate methods. Both approaches are beneficial when illustrating centrally mediated antinociceptive responses, which often focus on modifications occurring above the spinal cord level. The hot plate method is considered a supraspinal structured response, while the tail-flick technique mediates a spinal reflex to a nociceptive stimulus. The hot plate method produces two quantifiable behavioural responses about thermal discomfort duration. The paw-licking and jumping behaviours of rats are considered supraspinal integrated reactions. The OrthoGo formulation has demonstrated significant analgesic efficacy in both the methods.5, 6
Multiple studies indicate that the MIA led to the degradation of matrix proteoglycans, chondrocyte proliferation surrounding lesions, and cellular apoptosis in the affected regions. The histology findings of the untreated group revealed that MIA administration led to changes including articular cartilage degradation, synovial inflammation, and disintegration of the subchondral bone structure. Degenerative changes seen in the treated animals comprised chondrocyte degeneration, vertical fissures into the deeper layers, and surface fibrillation of the articular cartilage. The control rats that received intra-articular saline exhibited no alterations in the knee joint; specifically, the articular tissue structures, encompassing the synovial tissue, synovium, surrounding ligaments, tendons, tendon sheath, and subcutaneous tissues, maintained usual tissue architecture. Chronic inflammatory responses with tendon adhesion were noted in the animals injected with MIA.7, 8 The production of granulation tissue accompanied by neovascularization, edema, and mononuclear infiltration was seen. Additionally, chondrocyte necrosis accompanied by matrix degradation resulted in a reduction of articular cartilage thickness and facilitated the detachment of necrotic cartilage from the subchondral bone. In both the low dose and high dose of OrthoGo-treated rats, there was a significant reduction in inflammatory cell infiltration and cartilage damage. In both groups of rats, a few dispersed mononuclear cells were noted, and the cartilage thickness was elevated in comparison to the OA-induced group. Rats with meniscal injuries displayed unstable gaits that progressively became asymmetric post-injury, indicating reduced weight-bearing on the afflicted limb and the eventual onset of a slight limp. The stride length and base of support were utilized to assess the variation in gait. The OrthoGo formulation has achieved notable advancements and foundational support progress. The gait appears to progressively improve in the OrthoGo (including hemp seed powder) treated group.
This study results, along with those from earlier investigations, demonstrate that MIA replicates the neuropathic pain component associated with OA. Neuropathic pain is present in approximately 30% of patients. This OrthoGo formulation may be a beneficial therapy alternative for patients with neuropathic arthritis who are unresponsive to existing first- and second-line analgesics.9 Hemp seed cannabis has exhibited neuroprotective characteristics in many musculoskeletal disorders. OrthoGo containing hemp seed powder, glucosamine and collagen peptide supplements have a proven history of offering therapeutic benefits in the management of osteoarthritis.10 The OrthoGo formulation may serve as an effective method for mitigating functional deficits and improving performance in patients with osteoarthritis. The findings indicate that the OrthoGo formulation safeguards joints against cartilage degradation and inflammation, and also diminishes the progression of OA.
Conclusion
Considering the growing impact of osteoarthritis as a chronic degenerative condition on public health economics and patient well-being, it is imperative for interdisciplinary research teams, comprising primary care physicians, rheumatologists, orthopaedists, and physiotherapists, to prioritize the exploration of novel therapeutic alternatives. The OrthoGo formulation, comprising Hemp Seed, Glucosamine Sulfate Potassium Chloride, and Collagen Peptide, has emerged as a crucial component in the management of osteoarthritis due to its notable capacity to maintain joint structure, mitigate symptoms, cost-effectiveness, and absence of unwanted effects. Further research is required to fully understand this relationship; however, the OrthoGo formulation has been thoroughly shown to possess highly favourable risk-to-reward ratios, and it should remain a vital component of osteoarthritis treatment. The OrthoGo formulation increases pain and weight-bearing thresholds, diminishes cartilage degradation via stimulating osteoblast cell proliferation, and lowers the synthesis of pro-inflammatory cytokines and mediators in a rat model of osteoarthritis. We propose that the OrthoGo formulation positively influences inflammation and has potential as a supplement for individuals with osteoarthritis.