Introduction
Cancer remains one of the leading causes of morbidity and mortality worldwide, with its prevalence continuing to soar as the global population ages. As per the Global Cancer Observatory (GLOBOCAN) estimates, 2022 saw approximately 19.9 million new cancer cases globally. Triple-negative breast cancer (TNBC) represents a distinct subtype of breast cancer characterized by the absence of estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER2).1 This subtype constitutes around 10-15% of all breast cancer cases and is linked to aggressive tumor behavior, increased rates of metastasis, and a less favorable prognosis compared to other subtypes of breast cancer.2 TNBCs account for approximately 85% of all basal-like tumors. It is a heterogeneous disease with complex molecular features. 3
Figure 1
Comparison of triple-Negative breast cancer (TNBC) with other breast cancer subtypes. In the landscape of breast cancer, TNBC accounts for approximately 15% of cases,
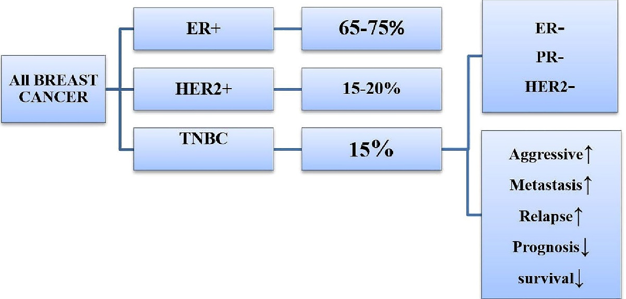
Characterized by its lack of estrogen receptor (ER) and progesterone receptor (PR) expression (ER-/PR-) and human epidermal growth factor receptor 2 (HER2-). Most breast cancer cases fall into the ER-positive (ER+) category, comprising approximately 65 to 75% of cases, where cancer cells express the estrogen receptor. The HER2-positive (HER2+) subtype constitutes approximately 15-20% of cases and is marked by the overexpression of the HER2 protein. TNBC stands out as a distinct subtype due to its unique molecular profile, making it particularly challenging to treat with targeted therapies commonly used for ER+ and HER2+ breast cancers. It is notorious for its aggressive nature, tendency to metastasize, high risk of relapse, and poor prognosis and survival outcomes.
Types and Subtypes of Triple-negative Breast Cancer (TNBC)
Breast cancer is a complex disease with a wide range of gene expression.4 The course of the disease, the outcome of treatment, and patient survival are all determined by this heterogeneity. Breast cancers are divided into two types depending on the presence or absence of the receptors. The presence of one or a combination of estrogen receptor (ER), progesterone receptor (PR), or human epidermal growth factor receptor 2 (HER2) makes some groups of breast cancer more susceptible to targeted hormone/HER2 therapy.5 TNBCs, on the other hand, are breast tumors that lack any of these three receptors.6 Therefore, receptor status has a significant impact on the treatment and prognosis of breast cancer patients.7 TNBC is the most aggressive group of breast cancers with a high recurrence rate and poor prognosis. However, each molecular subtype of TNBC responds differently to treatment and has different clinical outcomes.8 TNBC has been classified into six subtypes based on genomic expression profiling: basal-like 1 (BL1), basal-like 2 (BL2), immunomodulatory (IM), mesenchymal (M), mesenchymal stem-like (MSL), and luminal androgen receptor (LAR).9
Table 1
Subtypes of triple-negative breast cancer (TNBC) and their haracteristics
Pathways anomalies in breast cancer/TNBCs
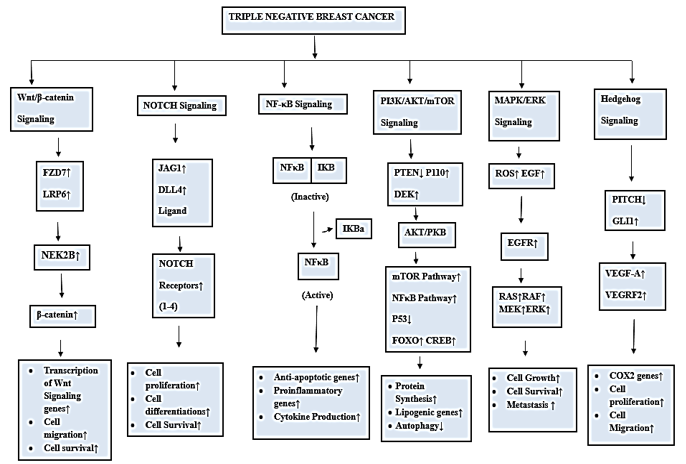
A crucial step in the initiation and development of cancer is cell signal transduction. Tumor cells display distinct features or hallmarks, such as uncontrolled cell growth, genomic instability, and resistance to programmed cell death. These characteristics arise due to modifications in various cellular signaling pathways, which contribute to the enhanced proliferation, advancement, and survival of tumor cells.10 These modifications occur as a result of mutations in oncogenes that lead to excessive protein expression, mutations in proteins that cause unregulated activity, or inactivation of tumor suppressor genes that facilitate these processes.11 As a result, cancer medications essentially target one or more of these signaling pathways. TNBC is associated with the dysregulation of various signaling pathways that contribute to its aggressive behavior and resistance to conventional therapies. Some of the key signaling pathways commonly de-regulated in TNBC are:
Wnt/β-catenin Pathway: Overactivation of this pathway, driven by genes like FZD7 and LRP6, promotes tumor growth and resistance in TNBC.12, 13, 14 Additionally, Nek2B, by interacting with β-catenin, further activates this pathway, leading to a worse prognosis. 15, 16 Targeting these components shows promise in preclinical studies.
Notch Signaling Pathway: High levels of Notch receptors (Notch1-4) and ligands (JAG1, DLL4) are linked to aggressive TNBC. Specifically, high NOTCH1 and JAG1 levels, especially when co-expressed, are associated with poorer survival. Inhibiting Notch1 and JAG1 is a potential therapeutic strategy. 17, 18
NF-κB Signaling: Dysregulation leads to increased expression of genes like CD44 and MMP9, promoting tumor invasion. Inhibiting NF-κB shows promise in reducing TNBC cell growth and invasiveness. 19, 20
PI3K/AKT/mTOR Signaling: This pathway is frequently dysregulated in TNBC, contributing to tumor growth and therapeutic resistance. This can occur through genetic alterations like PIK3CA mutations or PTEN loss.21, 22 Targeting this pathway shows promise; for instance, inhibiting DEK, an oncoprotein that regulates this pathway, reduces TNBC cell migration and angiogenesis.23 Additionally, using AKT inhibitors like ipatasertib has shown promise in clinical trials.24
MAPK (Ras/Raf/MEK/ERK) Signaling: High MAPK activity is linked to a poorer prognosis in TNBC. EGFR overexpression, common in TNBC, can lead to increased MAPK signaling, promoting tumor growth and hormone-independent proliferation.25 Additionally, reactive oxygen species (ROS), often elevated in TNBC, can further activate MAPK.26 Targeting this pathway, perhaps in combination with PI3K pathway inhibitors, is promising, as crosstalk between these pathways can lead to treatment resistance.
Genistein and TNBC
TNBC has a lower survival rate and is more likely to return and spread than other subtypes of breast cancer. Historically, chemotherapy was the primary treatment due to the absence of hormone receptors and HER2 amplification.27 However, such a therapeutic strategy is unable to overcome the negative side effects and medication resistance brought on by chemotherapy in cancer patients. Given this situation, investing efforts in researching and developing cutting-edge treatment drugs or interventional techniques that balance high effectiveness and low toxicity for individuals afflicted with TNBC becomes crucial.28 Plant-based chemicals are being studied as potential adjuvants or combination therapies for TNBC. Many bioactive compounds from medicinal plants have shown promising anticancer effects.29 Some of the most highly explored plant compounds having a wide range of therapeutic qualities, including antitumor action, are Flavonoids, carotenoids, alkaloids, and phenolics.30, 31, 32, 33
Among these phytochemicals, Genistein (GEN), also known chemically as 4,5,7-trihydroxy isoflavone, a natural isoflavone found in soybeans and legumes, is a promising phytochemical in cancer research. It has shown potential as a chemopreventive agent, inhibiting cancer cell growth and reducing the risk of certain cancers, particularly breast and prostate cancer in Asian populations.34 Genistein's impact on breast cancer is significant because it interferes with estrogen, a key player in cancer development, by binding to estrogen receptors (ER).35 It competes with 17β-estradiol, demonstrating a 4% binding affinity for ER-α and 87% for ER-β, making it a valuable component in hormone-related cancer treatment.36
Plants have served as natural remedies for centuries. 37 A diet rich in vegetables, fruits, and legumes contains high levels of antioxidants that protect against the harmful effects of free radicals that can lead to the development of cancer. 38, 39 TNBC accounts for approximately 15% of all breast cancers and cannot be treated with targeted drugs commonly used for other types of cancer treatment. Treatment options are limited due to the lack of targeted therapies compared to other types of breast cancer. Dietary GEN, a naturally occurring compound derived from soy and renowned for its flavone properties, has been postulated as the underlying catalyst for the remarkably low incidence of breast cancer among Asian women. 40
In vitro studies
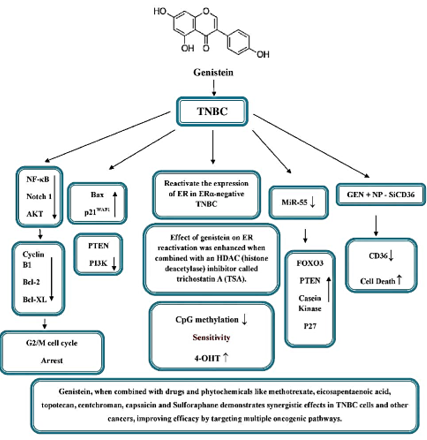
The MDA-MB-231 cell line is an invasive and aggressive breast cancer cell line that does not express ER, PR, or HER2. As a result, this cell line is known as a TNBC cell line. 41 There are few clinically effective treatments for TNBC. To better understand the molecular causes of this kind of breast cancer and to develop novel therapeutic approaches the MDA-MB-231 cell line is often used. In TNBC, GEN downregulates cyclin B1, Bcl-2, and Bcl-xL expression in MDA-MB-231 cells, potentially by inhibiting NF-κB via the Notch-1 pathway. SiRNA-mediated knockdown of Notch-1 or NF-κB also leads to downregulation of cyclin B1, Bcl-2, and Bcl-xL, resulting in G2/M cell cycle arrest and TNBC cell death. 42 Concentration-dependent treatment with GEN also led to the suppression of MEK5, total ERK5, and phospho-ERK5 proteins. This led to reduced NF-κB/p65 levels and DNA-binding activity, inhibiting cell growth and inducing apoptosis. Furthermore, GEN concentration-dependently reduced anti-apoptotic Bcl-2, increased pro-apoptotic Bax, and triggered caspase-3 cleavage and activity. 43 Gel shift assays revealed a cross-talk between NF-κB and Akt signaling pathways, with Akt cDNA transfection inducing NF-κB DNA-binding activity. Additionally, genistein counteracted EGF and Akt-induced NF-κB activation. These findings suggest that genistein's inactivation of NF-κB in MDA-MB-231 breast cancer cells is partly mediated by the Akt pathway.44
GEN was shown to inhibit the progression of MDA-MB-231 breast cancer cells, regulate apoptosis-related gene expression, and promote apoptosis via a p53-independent pathway. Upregulation of Bax and p21WAF1 might be molecular mechanisms by which GEN triggers apoptosis. 45 GEN treatment of MDA-MB-231 cells affects multiple cell cycle processes, including DNA replication, cohesin complex cleavage, and kinetochore formation. Additionally, it activates the DNA damage response, involving ATR and the BRCA1 complex. 46 GEN also shows strong binding to PTEN, an important player in the PI3K pathway in TNBC. When compared to the FDA-approved drug Olaparib using advanced analysis techniques, Genistein exhibits more favorable binding dynamics with PTEN compared to PTEN-Olaparib.47
Fatty acid oxidation (FAO) is vital for TNBC metabolism, fueling their growth, invasion, and metastasis.48 CD36, a key fatty acid receptor, plays a crucial role in this process. 49 GEN treatment reduces CD36 expression and activates p38 MAPK. Combining NP-siCD36 with lower GEN doses effectively hampers MDA-MB 231 cell growth, promoting cell death via the CD36/phospho-p38 MAPK pathway. 50 Furthermore, miR-155 a key player in regulating over 100 cancer-related pathways and cell cycle regulators, and its link to TNBC is well-established. 51, 52 Treatment with GEN has shown to decrease miR-155 expression in TNBC cells. This reduction triggers an increase in miR-155's target genes, including FOXO3, PTEN, casein kinase, and p27, leading to an anti-proliferative and proapoptotic effect in these cells.53
In vivo studies
In ERα-negative MDA-MB-231 xenograft mice, GEN could reactivate ER expression, and this effect was synergistically increased when paired with the HDAC inhibitor trichostatin A (TSA). The GEN therapy also restored ER-dependent cellular sensitivity to the antagonist tamoxifen (TAM) and activator 17-estradiol (E2). Additional research has shown that GEN can cause remodeling of the chromatin structure in the ER promoter, which aids in the reactivation of the ERα. 54 In vivo studies have also shown that lifelong GEN-rich diets, from conception onward, reduce CpG methylation in the BRACA1 gene in female mouse mammary glands. This leads to an increase in BRCA1 expression, restoring ERα expression in TNBC cells and enhancing sensitivity to antiestrogen 4-OHT.55
Moreover, co-administering GEN along with drugs like methotrexate, eicosapentaenoic acid, topotecan, centchroman, 56, 57, 58, 59 and additional combination with phytochemicals such as capsaicin and sulforaphane 60, 61 was observed to showcase remarkable synergy in terms of its impact on TNBC cells and various other forms of cancer. This remarkable revelation suggests that the efficacy of targeting multiple oncogenic pathways can be greatly enhanced by employing combinations of drugs rather than relying solely on the administration of a single monotherapy.
Figure 3
Different Molecular Targets of Genistein on TNBC. In vitro studies using the MDA-MB-231 cell line, a TNBC model, reveal that GEN downregulates key proteins (cyclin B1, Bcl-2, and Bcl-xL) involved in cancer progression, this effect is mediated via the NF-κB/Notch-1 pathway, resulting in G2/M cell cycle arrest and TNBC cell death. GEN inactivation of NF-κB is partly mediated by the Akt pathway. The upregulation of Bax and p21WAF1 seems to be the key molecular mechanisms behind GEN-induced apoptosis. Additionally, GEN shows strong binding to PTEN, an important player in the PI3K pathway in TNBC. Also, treatment with GEN has been shown to decrease miR-155 expression in TNBC cells. This reduction triggers an increase in miR-155's target genes, including FOXO3, PTEN, casein kinase, and p27, leading to an anti-proliferative and proapoptotic effect in these cells. GEN treatment lowers CD36 a key fatty acid receptor and activates p38 MAPK. Combining NP-siCD36 with GEN at lower doses effectively hinders MDA-MB 231 cell growth, triggering cell death via the CD36/phospho-p38 MAPK pathway. In ERα-negative MDA-MB-231 xenograft mice, GEN could reactivate ER expression, and this effect was synergistically increased when paired with the HDAC inhibitor trichostatin A (TSA). Lifelong GEN-rich diets in mice reduce BRCA1 gene methylation, increase BRCA1 expression, restore ERα expression in TNBC cells, and boost sensitivity to the antiestrogen 4-OHT. Lastly, Co-administering GEN with various drugs and phytochemicals shows synergistic effects on TNBC cells and other cancer types, emphasizing the potential of combination therapies.
Conclusion and Future Challenges
To this day TNBC remains a major challenge in breast cancer treatment. TNBC is a difficult subtype to treat due to its aggressive nature, lack of targeted therapy options, and high risk of recurrence and metastasis. 62 Despite these challenges, significant progress has been made in understanding the biology of TNBC and identifying potential therapeutic strategies. Research efforts have focused on exploring new therapeutic approaches such as immunotherapy, targeted therapy, and combination therapy. 63 In this review, we discussed the plant-derived compound GEN which have been demonstrated to have a strong anti-cancer effect in TNBC models in preclinical investigations. GEN primary molecular targets in breast cancer cells seem to be the NF-κB and Akt pathways.44 In vivo studies on mice also suggest that lifelong GEN-rich diets reduce methylation in the BRCA1 gene, potentially restoring ERα expression in TNBC cells and improving sensitivity to antiestrogen treatments.54 Moreover, combining GEN with various drugs and phytochemicals has shown synergistic effects against TNBC and other cancers, highlighting the potential of targeting multiple pathways simultaneously.64 This multifaceted approach holds promise for more effective TNBC treatment strategies. Studies have shown that Asian women who consume 20-50 times more genistein isoflavone-rich soy products per capita than their Western counterparts have a considerably lower risk of developing breast cancer.65, 66
In summary, Genistein holds promise for novel TNBC treatments, but challenges like dose formulation, bioavailability, interactions, and varying responses exist. To optimize its chemical efficacy, future research should explore mechanisms, conduct clinical trials, and investigate combination therapies. Collaboration between doctors, researchers, and patients is vital for advancing our understanding and treatment of TNBC, a highly aggressive form of breast cancer. Interestingly, nanoparticles may offer new prospective TNBC therapeutic options, and nano biosensors may be used as future TNBC diagnostics. Further research might guarantee that the efficacy of present medicines is monitored and that novel therapeutic options to treat TNBC, a disease with a bad prognosis, are discovered. This deep dive into the world of genistein has revealed its exciting potential in the fight against TNBC. While we've seen promising results in the lab, we need to bridge the gap to real-life patients. Robust clinical trials will be key to unlocking genistein's full potential, determining the ideal dosages, and understanding how it can best work alongside existing therapies. The journey ahead is filled with hope, and with continued research, genistein could become a powerful ally for those facing TNBC.