Introduction
Vaccine is the biological substance which when is administered into the body of a human being it boosts the immunity of the body. Vaccine increases that capacity of the body to produce several antibodies which are used to fight against diseases.1 When the antibodies are produced, the body will be able to protect itself against diseases for example tetanus, polio, mumps, tuberculosis, and COVID-19.2 Vaccination is an action of administering a vaccine into the body in order to strengthen the immunity of the body and production of antibodies. This concept of introduction of COVID 19 vaccine was the main approach of the scientist all over world wide to give protection against this SARS COVID 19 virus. A person who has received a vaccine can expose to a targeted disease without been infected.3 Immunization is the process by which a person becomes vaccinated hence been protected against a targeted disease. Immunization is done through vaccination. Immunity can be defined as the protection from an infection i.e. pandemic, epidemic diseases. It has evidences that if your body immunity system is strong to a disease you can be exposed to it without being infected. In this review we have tried to highlight the key information about vaccines and role of vaccines in this COVID 19 pandemic disease.4
Immunity
Human beings body have three types of immunity – natural immunity, acquired immunity and passive immunity. Other name for natural immunity is innate immunity. This is the type of immunity which a person is born with it.5 For instance, skin is a barrier for foreign bacteria or virus which protect our body from entering the foreign pathogens. Our immune system recognizes the foreign substances such bacteria whenever they inter into the body. Acquired immunity is possessed after our birth.6 Other name for acquired immunity is adaptive immunity. It is the kind of immunity which is developed when we are exposed to a disease or when we are immunized with vaccines. The acquired immunity is achieved through immunization. Passive immunity is achieved from another source and it lasts for a specific period of time. For instance, when baby consumes mother’s milk antibodies from mother’s body travel to baby through milk and provides temporary immunity to various diseases that a baby is exposed to. In immunity the major role is played by antibodies which are present in our body. On entry of any foreign agent in our body our body’s immune system gets stimulated and several antibodies are secreted against it to form antigen antibody reaction.7 The role of antibody in stimulating our immune system plays a very important role.8 Antibody is called immunoglobulin, it is protein in nature bearing Y- shaped tail portion which is used to bind with antigen to form antigen antibody reaction for foreign viruses, bacteria and pathogens. Antibodies are produced by specialized white blood cells known as B-cells or B lymphocytes. Antibodies attack antigens by binding to them and the antigen presenting cells showcase the antigen to the whole immune system.9 As soon as antigen binds to antibody through its head portion antigen antibody reaction occurs, B cells activates and divides and mature to form identical cells to form clones. There are five (5) classes of antibodies-(a) IgG: This type of an antibody has the highest opsonization and neutralization activities, and contributes to 70-75% of blood plasma. It is the most abundant antibody that is used to neutralize various antigens. This kind of an antibody detoxifies harmful substances and recognizes an antigen.(b) IgM: This kind of an antibody circulates in the blood and it accounts for approximately 10% of human immunoglobulins. It has a pentameric structure in which five basic Y –shaped molecules are linked together. B cells produced IgM first in response to microbial infections. IgM has less affinity than IgG but because of its pentameric structure it has higher avidity for antigens which enables it to bind to the cell surface receptor and activates cell signaling pathway.(c) IgA: This contributes to around 10-15% of the human antibodies. It is present in saliva, breast milk, nasal mucus, serum and intestinal fluids. Other two types of antibodies are IgE and IgD and both account for less than 1% of human antibodies.10
History
The historical literature of vaccine starts from the hundred’s years back by the ‘practice of immunization’. During 17th century Buddhist monks drank snake venom to develop immunity against poisonous snake bites.11 This was named as variolation. The father of vaccinology ‘Edward Jenner’ in 1796 developed the vaccine of smallpox by using attenuated strains of cowpox (vaccinia virus). The global eradication of smallpox was done by 1979-80. In this way vaccination proved an important precautionary treatment approach in eradicating dreadful pandemic diseases. Another scientist Louis Pasteur’s research was in the field of development of live attenuated cholera vaccine preparation and inactivated anthrax vaccine in humans by 1897-1900.12 Similarly plague vaccine and BCG vaccine was also invented by 19th century which still exists today. In 1923, Alexander Glenny used chemical formaldehyde to inactivate the toxins of tetanus. Similarly, by this method diphtheria vaccine was also invented by late 1926.13 The method of using whole cell in vaccines was used for development of pertussis vaccine and was the first licensed in US in 1948. Later in 1950-1985 viral tissue culture methods was used to develop inactivated polio vaccine (salk) and live attenuated oral polio vaccine (sabin). The polio myelitis infection was so serious that effected the whole world adversely and after mass polio immunization the success in eradication of this disease is done in many regions of the world.14
Table 1
Different vaccines invented
|
S.No. |
Vaccine |
Scientist who discovered |
Year of development |
Reference |
|
1. |
Small pox vaccine |
Louis Pasteur |
1798 |
History of vaccine book |
|
2. |
Tetanus vaccine |
Alexander Glenny |
1923 |
History of vaccine book |
|
3. |
Diptheria vaccine |
Emil Von Behring |
1926 |
History of vaccine book |
|
4. |
Pertussis vaccine |
Alexander Glenny |
1948 |
History of vaccine book 15 |
|
5. |
Polio vaccine |
Jonas Salk |
1950-1985 |
Vaccine and vaccinology book 16 |
|
6. |
Hepatitis B |
Dr. Baruch |
1965 |
History of vaccine book |
|
7. |
Hepatitis A |
Maurice Hilleman |
1979 |
History of vaccine book 15 |
Molecular genetics have been used from past two decades as a new insight in immunology, vaccinology, microbiology and others.16 Using the recombinant DNA technology method hepatitis B vaccines, pertussis vaccine with less reactivity and influenza vaccine are developed and achieving the maximum response. This recombinant DNA technology along with concepts of molecular genetics can be used in the development of new vaccines and delivery systems like viral vector vaccine, DNA vaccine.17 More effective vaccines need to be developed for tuberculosis, cytomegalovirus (CMV), herpes simplex virus (HSV), influenza, AIDS, respiratory syntical virus (RSV), SARS COVID 19 virus and others.18
Route of administration of vaccine
Route of administration is the way or path by which the vaccine or any other drug is admitted into a body and meet its desire of action. This is a critical factor for success of immunization. The following are the routes of administration of vaccine:19
Intramuscular injection (IM)
In this route of vaccine administration, the vaccine is introduced into the body through the muscle mass. The vaccines which use this route of administration are like vaccine for Hepatitis A, Hepatitis B, Infuenza, COVID-19, tuberculosis and malaria.20
Subcutaneous (SC)
In this route of administration of vaccine, the administer inject the vaccine into layer above the muscle and below the skin. The vaccine which are admitted into the body using this route are like vaccine for measles, varicella and yellow fever. Apart from the two routes of administration mentioned above, there are other two which are:21
Intradermal (ID)
In this the vaccine is administered on the most top layer of the skin. The example of vaccine which is administered into a body using this route is BCG for the ground that this route reduces the risk of neurovascular injury which could be caused by injection of BCG. Route of administration varies to maximize effectiveness of vaccine. Furthermore, manufacturers usually recommend the route of administration that limit best adverse reaction of the respective vaccine.22
Types of vaccines
There are several different types of vaccines but vaccine can be broadly classified by how the antigens are, how the antigen stimulates immune response against disease, how vaccines are prepared. Vaccines can be of different types.23
Live, attenuated vaccines
These vaccines are prepared by various methods like passing of disease-causing virus through series of various cell cultures or animal embryos. In animal embryos the virus is grown in various series of embryos in which the virus replicates faster in chick cells and becomes better and loses its ability to replicate in human cells.24 This concept can be implied for vaccines in which the virus does not replicate more in human body and when administered will not replicate enough but will only provoke immune system to provide protection against future infections.25
The following are the vaccines which are prepared by using live, attenuated virus; yellow fever vaccine, smallpox vaccine, chickenpox vaccine, rotavirus vaccine.
Inactivated vaccines
This kind of a vaccine is prepared using the killed version of a germ that causes a disease. This kind of a vaccine is not more potent than live, attenuated vaccine because inactivated vaccine usually does not provide protection that is strong as in live attenuated vaccines means that you need several doses over a time in order to get continuous immunity against diseases. The following are the vaccines which are prepared using inactive form of a germ: Hepatitis A vaccine, Polio vaccine, influenza vaccine and rabies vaccine. 26
Subunit, recombinant, polysaccharide and conjugate vaccines
These kinds of vaccines use specific pieces of a germ which includes its protein, sugar and capsid. These kind of vaccines gives a very strong immune system that is targeted to key parts of the germs. These vaccines also can be used to every one including those people with low body immunity compared to live attenuated vaccines which are highly recommended to those people wing strong body immunity. These vaccines are used to protect against the: Hepatitis B, Human papillomavirus (HPV) and Pneumococcal disease. 27
Toxoid vaccine
These vaccines use toxins of germs causing diseases to create immunity against the disease. This vaccine type includes examples: tetanus and diphtheria vaccines. 28
Viral vector vaccines
In this kind of a vaccine, a modified form of a virus is made in making these kinds of vaccines. Examples include vaccines for Ebola virus and SARS COVID-19 virus and HIV AIDS vaccine.
Messenger RNA vaccines
The short form of this vaccine is mRNA vaccine. Also, this vaccine is used to protect against COVID-19. These vaccines make viral proteins which stimulates immune response. Benefits of this kind of a vaccine includes time consuming means that mRNA vaccines take short period of manufacturing. The main reason for why this kind of a vaccine takes short period of manufacturing is because they do not contain a live virus. Another advantage of mRNA vaccine is that, there is no risk of causing disease in the person getting vaccinated. 29
Mechanism of action of vaccine
When the pathogen weakened or inactivated enters our body through vaccines, they stimulate our immune system by the Antigen Presenting Cells (APC’s). These APC’s showcase the pathogen to whole immune system which activates major histocompatibility tissues (MHC II) and activates the thymus gland to produce specific B cells and T cells. B cells play major role by developing antibodies against that pathogen and making antibodies in our body.30 Memory B cells containing antibodies remains stored in our immune system which protects our body from further entry of this virus in our body and provides immunity.
The administered vaccine confers the body immune cells to quickly recognize, react and subdue the disease-causing agent. When the body is exposed to same disease-causing agent, the immune system activates memory B cells and eliminate the infection before it produces damage to the body. The efficiency and efficacy of vaccines depend on nature of constituents in vaccine and how our immune system process it. Some virus changes their strains and require immunization again and again in years. Examples includes influenza virus, SARS COVID 19. In children’s the thymus gland is not mature enough and is less capable of producing memory B cells hence in their body the duration of this vaccines can be shorter.31
Vaccine for SARS Covid 19
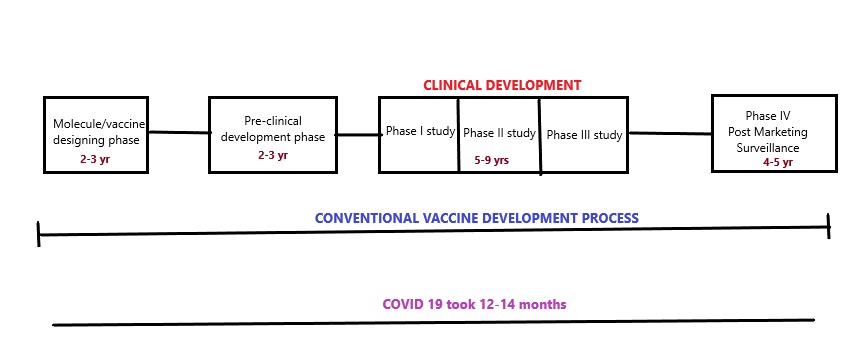
In the history of vaccines, the emergence in COVID 19 vaccines is very fast and at unpredictable speed. Due to the tendency of the spread of COVID 19 and the rate of death it caused there was immense need in the development of COVID 19 vaccine for fast immunization to protect the world from the pandemic situation. Many pharmaceutical companies and research laboratories from all over worldwide continuously worked in the development of COVID 19 vaccines. As per the data available around 184 vaccines are in preclinical development phase and 104 vaccines are in clinical drug trials.32 Around the world around 18 COVID 19 vaccines are in use which are approved by the government. The conventional vaccine development process takes around 15-16 years to develop a successful vaccine but in this COVID 19 scenario the researchers within 12-14 months launched COVID 19 vaccines with potential benefits. However, the intense study needs to be done on the effectivity of these vaccines in developing strains of this virus.33
Table 2
Covid 19 Vaccines are developed in four categories
Whole virus vaccines
This vaccine uses attenuated or inactivated form of SARS COV-2 to activate immunity against COVID 19 infections. These viruses do not cause illness and replicates in the body to stimulate immune system only. Weakened vaccines contains viral genetic constituent which has been destroyed chemically or heat or radiation so that they are capable of only stimulating immune system.34 These types of vaccines help in developing cellular immunity however there is certain limitations to the usage of this type of vaccines. The vaccine may be excreted in the faecal material and can cause transmission of SARS CO V 2 in non-immunized individuals. These vaccines are approved for emergency use.35
Protein based vaccines
These vaccines contain viral antigenic fragments which are produced by rDNA technology. They are safe and well tolerated as compared to whole virus vaccines. The vaccines have low immunogenicity which can be improved by combining with some adjuvants.
Viral vector vaccines
Virus has main property that they multiply rapidly in their invading host cells and makes new viruses by using the genome of host. The virus is capable enough to stimulate the immune system. In this vaccines host cells receives message or code to make particular antigens. Viral vector acts a medium to deliver SARS CoV 2 viral code into host cells. The virus is made inactive by chemical treatment and do not cause disease. Virus that were used as vectors earlier before invention of SARS CoV 2 vaccine were adenovirus, vaccinia virus and measles virus.36 The viral vaccine for Ebola virus disease was first to be used for human population before SARS CoV 2. In this way viral vaccines can be used to stimulate immune system without causing disease. The limitation of this vaccine is the host body is earlier exposed to virus and immune system gets stimulated then this vaccine becomes ineffective.
Nucleic acid vaccines
This vaccines contain genetic information in the form of DNA (deoxyribonucleic acid) or RNA (ribonucleic acid). These vaccines are prepared by using principles of recombinant DNA technology. The vaccine uses the DNA of antigen which is inserted to bacterial circular DNA- plasmids. 37 Plasmids replicate and transfer the codes of antigen in the cells and translate the antigen code to proteins. Using RNA it basically uses mRNA which is then used to translate proteins in the cells. Now these nucleic acids when enters the host body it stimulate the immune response which involves T cells and B cells to produce antibodies against the viral antigen and protect the host body. 38 As off 12 January 2022 data of World Health Organization have listed below mentioned COVID 19 vaccines in terms of safety and effective use along with the emergency use.39
As the vaccine was launched for SARS CoV 2 COVID 19 the vaccination program was initial started for old age group of people (45 years and above). The vaccination started in 2021 as a major step to defeat COVID 19 virus. Later the adults (18–45 yrs) were benefited by the program and recently the program has been launched for teenagers (14-18 yrs). The vaccination or immunization has major impact in controlling the widespread of such effective COVID 19 virus. Although there are some challenges are there which proves some ineffectiveness of these vaccines in the developing and growing strains of this virus. The booster doses of the vaccines are been given to the population to maintain the process of immunity for a longer period of time.40
COVID 19 vaccination program was at maximum peak later by end of 2021. A lot of people were benefitted first with single dose of vaccine and later the second doses were given due to shortage off supply. The countries who gave vaccination to 50% people included United Kingdom, Chile, Israel, Bahrain, India, France, Germany, United States of America and many more. 41
Conclusion
More advancements is needed and using higher technologies scientist from all over world trying to develop more effective COVID 19 vaccine which is effective in circulating strains worldwide. World Health Organization played a very pivot role in delivering all the information of COVID 19 vaccine research that is carried by different companies whole worldwide. Vaccine developed by heat treatment could be researched for better results. Despite the circulating strains of this virus the immunization and its booster doses should be given to stop the widespread of this COVID 19 virus. In getting won over this pandemic the world should unite and help each other to eradicate this pandemic.