Introduction
Lip balm or lip salve is a wax-like substance applied topically to the lips to moisturize and relieve chapped or dry lips, angular cheilitis, stomatitis, or cold sores. Lip balm often contains beeswax or carnauba wax, camphor, cetyl alcohol, lanolin, paraffin, and petrolatum, among other ingredients. Some varieties contain dyes, flavour, fragrance, phenol, salicylic acid, and sunscreen.
Lip balm can be applied where a finger is used to apply it to the lips, or in a lipstick-style tube from which it can be applied directly.
Lip balm was first marketed in the 1880s by Charles Browne Fleet, 1 [unreliable source?] though its origins may be traced to earwax. 2 More than 40 years prior to the commercial introduction of lip balm by Fleet, Lydia Maria Child recommended earwax as a treatment for cracked lips in her highly-popular book, The American Frugal Housewife. Child observed that, "Those who are troubled with cracked lips have found this earwax remedy successful when others have failed. It is one of those sorts of cures, which are very likely to be laughed at; but I know of its having produced very beneficial results." 3
The primary purpose of lip balm is to provide an occlusive layer on the lip surface to seal moisture in lips and protect them from external exposure. Dry air, cold temperatures, and wind all have a drying effect on skin by drawing moisture away from the body. Lips are particularly vulnerable because the skin is so thin, and thus they are often the first to present signs of dryness. Occlusive materials like waxes and petroleum jelly prevent moisture loss and maintain lip comfort while flavorings, colorants, sunscreens, and various medicaments can provide additional, specific benefits. Lip balm can be applied where a finger is used to apply it to the lips, or in a lipstick-style tube from which it can be applied directly.
Antioxidants
Antioxidants are compounds that inhibit oxidation. Oxidation is a chemical reaction that can produce free radicals, thereby leading to chain reactions that may damage the cells of organisms. Antioxidants such as thiols or ascorbic acid (vitamin C) terminate these chain reactions. To balance the oxidative stress, plants and animals maintain complex systems of overlapping antioxidants, such as glutathione and enzymes (e.g., catalase and superoxide dismutase), produced internally, or the dietary antioxidants vitamin C and vitamin E.
The term "antioxidant" is mostly used for two entirely different groups of substances: industrial chemicals that are added to products to prevent oxidation, and naturally occurring compounds that are present in foods and tissue. The former, industrial antioxidants, have diverse uses: acting as preservatives in food and cosmetics, and being oxidation-inhibitors in rubber, synthetic plastics, and fuels. 1
Antioxidant dietary supplements have not been shown to improve health in humans, or to be effective at preventing disease. 4 Supplements of beta-carotene, vitamin A, and vitamin E have no positive effect on mortality rate 5, 6 or cancer risk. 7[needs update] 8 Additionally, supplementation with selenium or vitamin E does not reduce the risk of cardiovascular disease. 9
Vitamin C
Ascorbic acid or vitamin C is a monosaccharide oxidation-reduction (redox) catalyst found in both animals and plants. 10 As one of the enzymes needed to make ascorbic acid has been lost by mutation during primate evolution, humans must obtain it from their diet; it is therefore a dietary vitamin. 10, 11 Most other animals are able to produce this compound in their bodies and do not require it in their diets. 12 Ascorbic acid is required for the conversion of the procollagen to collagen by oxidizing proline residues to hydroxyproline. In other cells, it is maintained in its reduced form by reaction with glutathione, which can be catalysed by protein disulfide isomerase and glutaredoxins. 13, 14 Ascorbic acid is a redox catalyst which can reduce, and thereby neutralize, reactive oxygen species such as hydrogen peroxide. 15 In addition to its direct antioxidant effects, ascorbic acid is also a substrate for the redox enzyme ascorbate peroxidase, a function that is used in stress resistance in plants. 16 Ascorbic acid is present at high levels in all parts of plants and can reach concentrations of 20 millimolar in chloroplasts. 17
Beets contain an antioxidant called alpha-lipoic acid. This compound may help lower glucose levels and increase insulin sensitivity.
However, most of the doses in these studies were far higher than those that are available in beetroot. The effects of smaller dietary doses are not yet clear from the available research.
Beetroot can also add the following vitamins and minerals to a person’s diet.
Table 0
|
Nutrient |
Percentage of an adult’s daily requirement |
|
Vitamin C |
7.4% |
|
Vitamin A |
0.3% for males, and 0.39% for females |
|
Folate |
37% |
|
Magnesium |
7.83% for males, and 10.97% for females |
|
Phosphorus |
7.77% |
Beets also contain small amounts of:
Green, leafy vegetables such as beet tops provide high levels of dietary nitrate. Cooked beet tops are a great source of iron, vitamin C, vitamin A, magnesium, potassium, and folate.
Materials and Methods
Beet root was peeled and blended in a mortar and pestle and filtered. To the filtrated add few drops of coconut oil and triturated well and kept in freezer for 24 hours. Lip balm was prepared and this was futher used for the analyis of antioxidant activity.
Method of total antioxidant activity was determined by PFRAP, CUPRAC and Fluorimetry method FRAP Antioxidant reaction with a Fe(III) complex
PFRAP
Potassium ferricyanide reduction by antioxidants and subsequent reaction of potassium ferrocyanide with Fe3+ Colorimetry.
CUPRAC Cu (II) reduction to Cu (I) by antioxidants.
Fluorimetry
Fluorescence is the emission of light by a substance that has absorbed light or other electromagnetic radiation of a different wavelength. In most cases, emitted light has a longer wavelength, and therefore lower energy, than the absorbed radiation. Fluorescence emission occurs when an orbital electron of a molecule relaxes to its ground state, by emitting a photon of light after being excited to a higher quantum state by some type of energy. Fluorescence assay has been used to antioxidant content determination. The emission spectra were obtained under excitation at about 310 nm and fluorescence in the 320-800 nm range was evaluated.samples without BHA and TBHQ showed fluorescence band at about 420nm, which can be attributed to tocopherols, inherent to the vegetable oils used in the biodiesel production. The addition of BHA and/or TBHQ is responsible for the occurence of a fluorescence band around 330nm.
Table 1
Results of emission spectra
Results and Discussion
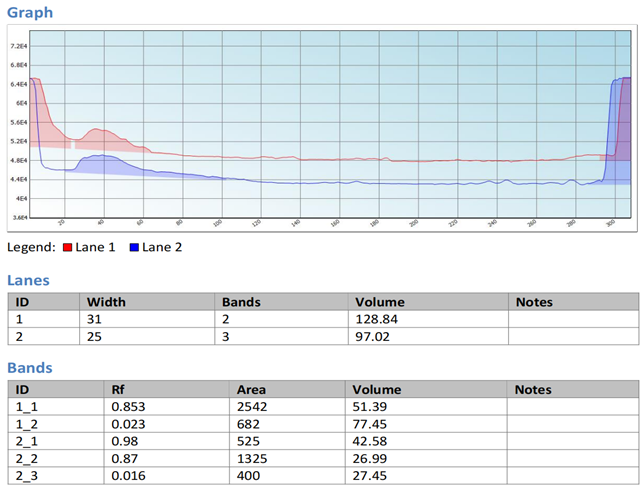
PFRAP, CUPRAC, Flourimetry methods and results of emission spectra was examined and fournd to possess almost same concentration of 0.3 ug/ml. And the ascorbic acid was determined by HPTLC method and the Rf value of the lip balm was found to be 0.98 and is near to the abscorbic acid sample.
Conclusion
PFRAP, CUPRAC, Flourimetry methods and results of emission spectra was examined and fournd to possess almost same concentration of 0.3 ug/ml. And the ascorbic acid was determined by HPTLC method and the Rf value of the lip balm was found to be 0.98 and is near to the abscorbic acid sample. And hence proved that beetroot lip balm was found to possess antioxidant activity. The anti oxidant activity was determined and was found to be simple, novel and economical.
Acknowledgements
I am grateful to the management of SIMS College of Pharmacy, Mangaldasnagar, Guntur, without which our work would not be complete.