Introduction
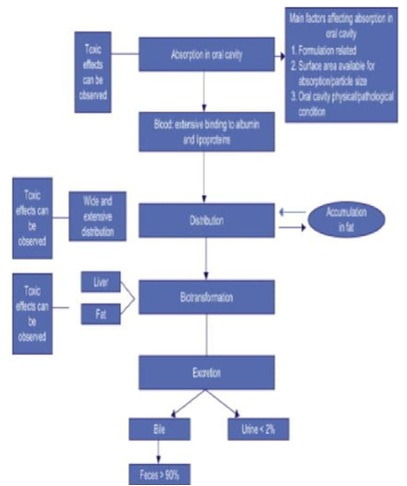
Toxicokinetics refers to the study of absorption, distribution, metabolism/biotransformation, and excretion (ADME) of toxicants/xenobiotics in relation to time. Absorption describes the entrance of through the air, water, food, or soil. Once a chemical is inside a body, it can be distributed to other areas of the body through diffusion or other biological processes.1
In exposed organisms, metabolism is an important factor in determining the bioaccumulation, fate, pharmacokinetics (or toxicokinetics), and toxicity of contaminants.2
Contaminant exposure can results in the induction of Phase I cytochrome P450 monoxygenase (CYP) enzymes and Phase II conjugation enzymes (e.g- glucuronosyl transferase, sulfotransferases, and gluathione-S-transferases).3
Knowledge of toxicokinetics is for evaluating toxicity or for selecting dosages to be administered to animals in toxicology studies. A goal of most toxicity research is to infer expected human health risks from the results in test animals are commonly exposed to extremely high concentration of test chemicals, by unusual routes of administration, for their entire life time.4 The challenge is to extrapolate from animal results to predict effects in human population that have very different exposure patterns.
Toxicokinetics involves the generation of kinetic data to assess systemic exposure, either as an integral component of preclinical toxicity studies, or in specially designed supportive studies.1 These data help to understand the relationship between observed toxicity and administered dose. They also play a role in the clinical setting, assisting in the setting of plasma limits for early human exposure and in the calculation of safety margins.5
The primary objective of toxicokinetics is to describe the systemic exposure achieved in animals and its relationship to dose level and the time course of the toxicity study.6 Toxicokinetics is largely reflective of the ADME of a molecule as it moves through the body of an organism. As with all animal studies, interspecies differences in ADME should be considered prior to clinical human testing and during data extrapolation.7 If correctly utilized, Toxicokinetics studies can provide quantifiable endpoints to better explain negative effects that may arise during toxicity studies6.
Toxicokinetics/Pharmacokinetics
All drugs under development to treat human diseases must undergo a series of, Toxicokinetics/Pharmacokinetics investigations in animals and humans to gain an understanding of the their properties. 8
Toxicokinetics are shares important parameters, like Cmax and AUC, with preclinical Pharmacokinetics, the studies are:
The primary goal of TK is to correlate findings of toxicity (not therapeutic efficacy) with a corresponding level of exposure to an experimental drug compound.
The TK arm of a nonclinical toxicology study generally has fewer time points, fewer subjects and fewer endpoints compared to nonclinical and clinical PK studies.
Half-life (t1/2) may not be accurately determined in TK studies (although it is frequently estimated) due to relatively sparse sampling of blood or plasma for concentration-time analysis. 9
Toxicokinetics
Clinical veterinary toxicology
Pharmacokinetics and toxicokinetics are also important concepts regarding food residue and safety issues as well as drug withdrawal times in food-producing animal. 10 In 1982, the U.S. Department of Agriculture (USDA) Extension Service began involvement in an educational/service project to further enhance the safety of animal-derived foods. The Residue Avoidance Program, which was initially founded by the USDA Food Safety and Inspection Service (FSIS), targeted the area of xenobiotics residues. 11
Models and methods for in vitro toxicity
Toxicokinetics study is essentially required to related the dose or chemical concentration and the mode of action of the chemical and its various metabolites. The toxicokinetic process is responsible for the distribution and formation of various chemical entities at the target tissue, which is further responsible for determining the dose at toxicological site. 12 The basic toxicokinetic parameter is based on in vitro and in silico studies, which detects the potential of accumulation and the potential of distribution or inhibition of chemical in the tissues/organs. 13
In vitro approaches retrieve information of prime importance in the area of toxicokinetic studies. One of the representative model, the physiologically based toxicokinetic model, can be obtained by including the kinetics of metabolism by the liver and any other organ capable of biotransforming. 14
Preclinical drug development
Toxicokinetics is the generation of pharmacokinetic data as a part of various toxicity studies in order to assess systemic exposure. 15 The measurement of peak and total exposure in these studies helps to determine the relationship between the toxicological effects and the exposure. The data on exposure is more relevant for comparing effects in animals and man. This is because the pharmacokinetics of a drug varies extensively between the species. 16
These studies generally include repeated-dose toxicity studies, reproductive, and carcinogenicity studies. Since, single dose studies are usually done before a bio-analytical method has been developed, toxicokinetic monitoring cannot be integrated in these studies. 16
Normally, in the case of large animals, blood samples for the generation of toxicokinetic data are collected from main study itself. However, in the case of smaller species satellite group may be required. ICH Guidance S34 provides detailed recommendation on toxicokinetic assessment. 17
Toxicity and safety evaluation of pesticides
Toxicokinetics studies provide data on the ADME of the chemical pesticide. In general terms, how does it enter the body, where does it go, and what happens to its. These studies will also provide information on other parameter of interest, including difference between small and large doses, and single versus multiple exposures. 18
Understanding the toxicokinetics of the pesticide may also enable more appropriate selection of doses and routes of administration used in many of the laboratory studies, as well as toxicokinetic consideration, will assist in determining the appropriate route of exposure and duration of study.
Toxicity and Safety Testing
Metabolism and Toxicokinetics
Toxicokinetics describes what an animal's body does with a drug or chemical or its metabolites following administration of the parent drug, typically at a high dose relative to the therapeutic on or effective dose. The 1994 ICH S34 guideline identifies toxicokinetics as an integral part of a nonclinical testing program because it supports species selection and the treatment regimen, enhances the toxicity data, and provides comparisons with clinical data, which is important in assessing risk and safety in humans. The guidance also notes the role for toxicokinetics in interpretation of similarities and differences across species, which may result from difference in protein binding, tissue uptake, receptor properties, and metabolic profiles. 19
Toxicokinetics from in vitro studies contributes to understanding mechanisms of action and species differences. For example, Carney et al. (2008) used WEC to ethylene glycol (EG) is teratogenic in rats but not rabbits. Maximal levels of unchanged EG in rabbits were comparable to those reported for pregnant rats while maximal levels of the teratogenic metabolite, glycolic acid, in rabbit maternal blood and embryo were 46% and 10% of the respective levels in rats. 20
Modeling Toxicity
Toxicokinetics Modeling
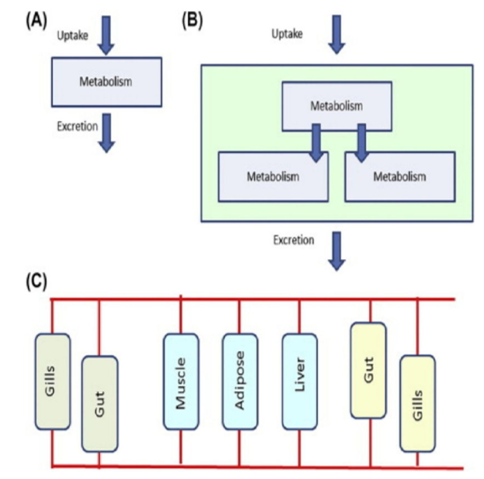
Toxicokinetic models integrate the uptake, metabolism, and excretion of chemicals in an individual organism. 20 The major aim of the modeling is to get the internal effective concentration (IEC), in the simplest case, the organism is considered as a single compartment, and both the uptake and the excretion of chemicals can be thought of as simple exponential uptake and loss. 21 Mathematical modeling of multi-compartment, The compartmental models require only limited time-series data for a compound in a reference compartment to allow estimation of pharmacokinetic parameters, the data are collected from different tissues/physiological compartments and their perfusion is take into account, the compartments are chosen so that they represent physiologically relevant entities. 22
The single-compartment model
The multi-compartment model. In multi-compartment models, the calculation of uptake and excretion become complex. The different compartments are defined operationally and have no preconceived notions about physiological relationships.
Physiological based toxicokinetic (PBTK) models divide uptake, metabolism, and excretion (signified in the figure by different colors) into functionally relevent compartments. 23
Orally delivered nanoparticle drug delivery systems for dental application and their toxicity on system organs
Principles of Toxicokinetics
Toxicokinetics is the mathematical description of the uptake and disposition of a chemical in the body. Toxicokinetics modeling is usually implemented by describing the time course of the amount or concentration of the parent substance and its metabolites in one or several body compartments.24 Toxicokinetics models may also be used to strengthen experimental results by linking data obtained under different experimental conditions in uniform model. 25 One might, for example, use a single model to explain the toxicokinetics after different routes of administration or to describe uniformly the toxicokinetics of a family of related chemicals.26 Toxicokinetics modeling may also be used as an aid in the development of biological exposure monitoring. 24
Some may consider that pharmacokinetics is all to do with arithmetic and others that toxicology is only concerned with chemicals that make us sick. In fact, neither discipline can develop without the other, especially not understanding the mechanisms of toxic reaction.27
Toxicology Testing
Toxicology programs have evolved from a traditional exploration of chemistry and applied toxicity of chemicals and drugs to a more comprehensive study of toxicology and toxicology testing as independent entities. Toxicology testing starts with basic toxicological principles, including absorption, distribution, metabolism, and elimination of toxins, including chemical and drug.28
This introductory material is useful in understanding the applications of toxicology testing. The fundamental principle of toxicology testing in animals in greater detail. This section explains acute toxicity studies as well as subchronic and chronic studies performed on animal.29
Special emphasis is placed on study design and determination of classical indicators for acute and chronic testing, such as the LD50. 30
Applications of Toxicokinetics
Toxicokinetics, toxicodynamics and toxicogenomics method are the potential tools in human health risk assessment.31 Application of these techniques give detail knowledge about the drug kinetics and metabolism; improved assessment strategy with greater efficiency, use fewer animals and provide better data for risk assessment purpose; rescue at-risk programs in preclinical/early clinical development; proactively screen/evaluate leads at early stages using predictive tools for toxicity and mechanism of action, develop preclinical biomarkers of drug response and toxicity, adoption of toxicity management approaches to improve the therapeutic outcomes.32
Studies reveal the drug toxicity at molecular as well as genetic level that helps researchers and physicians to reduce the undesired effects of drug.TK/TD/TG approaches are very important to develop experiments designed to understand the molecular basis of drug toxicities.31
Toxicokinetics evaluation is important in drug development stages. This evaluation should constitute effective analytical methods having good accuracy and precision, adequate sampling, drug and metabolites evaluation both in animals and humans and sufficient results evaluation.33
Toxicokinetics data is important to know the toxic responses to that of drug in preclinical which can be used to set safe dose for clinical use of new drugs. It also gives support to mode of action analysis and extrapolation across exposure routes.34 Toxicokinetics used in other areas of pharmacokinetics which act as a biomarkers for doing screening studies which provide data for allometric species scaling, measuring drug levels in non-plasma samples (tissues, urine and bile). Even though toxicokinetics evaluation is only a small part of the process of understanding the fate of a drug, it has a vital part in drug development.35
The evolution of toxicity whether as discrete or sequential, single or multiple events, each effects measured or observed can be identified by toxicokinetic data.36
The route of administration and the nature of the formulation to be used in humans, as well as the therapeutic indication for which the compound is intended can also be detected.35
Conclusion
Toxicokinetics studies is to describe the systemic exposure achieved in animals and its relationship to dose level and the time course of the toxicity study. Increased implementation of toxicokinetic sampling in all stages of toxicity testing could provide significant improvement in terms of efficiency, relevance, reliability, time constrains and budget.