Introduction
Pharmacovigilance (PV) is defined as the science and activities relating to the detection, assessment understanding and prevention of adverse effect or any other drug related problem. WHO established Programme for International Drug Monitoring (PIDM) in 1968, after Thalidomide disaster in 1961. The WHO Collaborating Centre for International Drug Monitoring also known as Uppsala Monitoring Centre (UMC) was established in 1978 in Sweden. Currently, 134 countries are part of the WHO PV Programme.1 Globalization of the pharmaceutical industry has prompted efforts towards harmonisation of PV practices worldwide to enable improved knowledge of medicine's benefit-risk profile and risk communication.2 The International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) is a unique body bringing together regulatory authorities and pharmaceutical industry to discuss scientific and technical aspect of ADR monitoring. ADR are undesirable effects that occur as a result of drug treatment at doses normally used in man for treatment. Although there are many terms indicating the harmful and undesirable effects of drug treatment, the term ‘adverse drug reaction’ describes it most appropriately. ADRs are a common cause of concern to both the physicians and the patients. They not only add to the spiraling costs of disease treatment, but also major cause of morbidity and mortality. However, many observed ADRs go unnoticed in various countries including India. In India, there are certain peculiarities of drug use such as: large number of patients, lower doctor-patient ratio, and self-medication practice, drugs of alternative systems of medicine, malnutrition, presence of counterfeit drugs, and presence of the highest number of combinational products. Therefore, incidence of the adverse drug reactions in India is likely to be same as that of the developed nations, or even more. Inspite of presence of organised ADRs Monitoring Centres (AMC), the number of reports sent annually are less than anticipated. These calls for the urgent need to give a boost to the monitoring of adverse reactions through public education against self-medication, introduction to drug-safety in the curriculum of medical and pharma undergraduates, a systemic and periodic training of health professionals. Multi-prolonged strategy can lead to reduction in the incidence of under-reporting.3, 4, 5
Methodology
The method involved the review of research articles, review articles and other materials from the internet sources. Various journals, articles and reports were thoroughly searched for the analysis of the under reporting of adverse drug reactions in different countries. The information obtained helped to understand the scenario and history of Pharmacovigilance in India and also the scenario in different countries. Various factors leading to the under-reporting of ADR were also identified in the process.
Adverse Drug Reaction (ADR) reporting scenario in some countries including India
Pharmacovigilance is an integral part of standard healthcare process, but it is still not widely accepted in India. In various ways, it has been established that ADR causes considerable amount of morbidity and mortality.3 Total number of ADRs reported in India is very few. This is because it is still in a developing phase. As any other developed country in the world, India felt the need of drug safety monitoring. The origin of pharmacovigilance in India goes back to the year 1986, when officially an ADR monitoring system consisting of 12 regional centres was proposed with each centre covering a population of 50 million. However, there was no progress made till 1997 when India joined the WHO Adverse Drug Reaction Monitoring
Programme managed by Uppsala Monitoring Centre (UMC). This attempt was again unsuccessful and from November 2004, Government of India launched National Pharmacovigilance Program for India (NPPI) supported by WHO and World Bank.
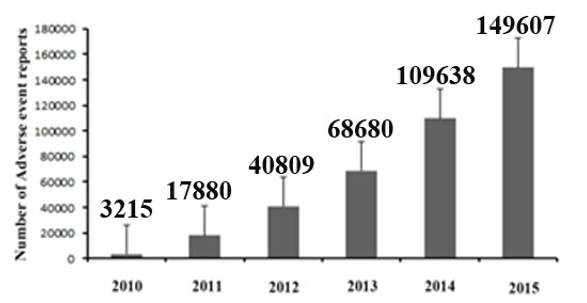
The NPPI was to be overseen by the National Pharmacovigilance Advisory Committee (NPAC) based in the Central Drugs Standard Control Organization (CDSCO), New Delhi. Two zonal centres - the South-West zonal (SW) centre and the NorthEast (NE) zonal centre were responsible to collate information from all over the country and send it to the Committee as well as to UMC. Under these 2 zonal centres there were 5 regional centres and under 5 regional centres there were 26 peripheral centres. However this program also did not meet up with desirable result. Recognizing the need to restart the National Pharmacovigilance Programme (NPvP), jointly formulated by the Department of Pharmacology, AIIMS and CDSCO in late 2009. After understanding the need for a better ADR reporting system in India, the health ministry launched a programme called Pharmacovigilance Programme of India (PvPI) in the year 2010.4 Under this programme, multiple Adverse Drug Reaction Monitoring Centres (AMC) were set up across various cities in India, in all the medical colleges approved by Medical Council of India (MCI) with the AIIMS, New Delhi as the National Coordination Centre (NCC) for monitoring ADRs in the country for safe-guarding public health. In the year 2010, 22 ADR monitoring centres including AIIMS, New Delhi was set up under this programme. To ensure implementation of this programme in a more effective way, the NCC was shifted from the AIIMS to the Indian Pharmacopoeia Commission (IPC), Ghaziabad, Uttar Pradesh on 15 April 2011. Currently, there are around 170 Adverse Drug Reaction Monitoring Centres (AMC) in India. The main function of these AMCs is collection of ADR reports and uploading them in VigiFlow database. Over the last 5 years the National Co-ordinating Centre (NCC) has played an important role in generating awareness for HealthCare Professionals (HCP) on the importance of reporting ADRs. This initiative helped to generate around 150,000 reports by the end of 2015.6, 7 However, Indian AMC functional rate was very low compared to the developed nations. Lack of awareness, training and knowledge were marked as key factors affecting the Pharmacovigilance programme of India (PvPI).
Adverse Drug Reaction (ADR) reporting scenario in USA
In USA Food and Drug administration (FDA) has the main responsibility to ensure drug safety. However, the US FDA relies on spontaneous reporting of Adverse Drug Reactions. Studies have shown that under-reporting of ADR is widespread 7, 8. FDA. Adverse Drug Event Reporting System (FAERS) was developed to give information related to human ADRs reported to FDA adhering to ICH (International Conference on Harmonisation) guidelines. Like any other standard ICSR database duplicate, incomplete and unverified reports are the main drawbacks of FDA Adverse Event Reporting System (FAERS).
Adverse Drug Reaction (ADR) reporting scenario in Malaysia
All reports of ADR associated with the use of registered product occurring in Malaysia must be reported to Drug Control
Authority (DCA) within the stipulated timeline. Registration holders who have registered a product containing a New Chemical Entity (NCE) after 1-Jan-2002 must routinely submit Periodic Safety Update Reports (PSUR) on the product for first 2 years post approval in Malaysia and then annually for subsequent 3 years.9
Adverse Drug Reaction (ADR) reporting scenario in Nepal
Nearly 75% drugs in Nepal are imported from foreign countries as Nepal has limited capacity in terms of drug manufacturing. Prior to marketing a drug, Department of Drug Administration (DDA), the regulatory authority, thoroughly assesses the drugs on the basis of the data available from other countries. Pharmacovigilance activities are in a preliminary developing stage in Nepal. ADR reporting is mainly confined to the healthcare professionals. 10
Adverse Drug Reaction (ADR) reporting scenario in Bangladesh
In Bangladesh Director General of Drug Administration (DGDA) is plays an active role to ensure safety of patient 11. In 1996, under guidance of WHO, a cell was established in DGDA. In 1997, Ministry of Health and Family welfare formed ADR Advisory Committee (ADRAC) to evaluate, analyse and make recommendation for solving problems of medicinal hazards due to ADRs.
Adverse Drug Reaction (ADR) reporting scenario in Pakistan
The DRAP has established Pakistan National Pharmacovigilance Centre (PNPC). Pakistan has limited resources in the system of healthcare system. Awareness level on ADR reporting was measured among physicians (51%), pharmacists (29.7%) and Nurses (19.3%). The results showed that 65.5% of HCP population observed ADRs amongst them only 57.4% were reported these in their respective hospitals.12 The number of deaths related to ADR is not known due to lesser developed process of pharmacovigilance. The pharmacovigilance system of Pakistan is still at its infancy, and many reforms have been introduced by the government body to improve the system.13
Table 1
Comparison ofPharmacovigilance System in India and few other countries.
Factors leading to under-reporting of ADRs in India
Under-reporting of ADRs is a major setback in the evaluation of the safety profile of a drug. Various studies have been done to understand the reason behind under-reporting of ADRs. The major causes of under-reporting are:
Lack of proper knowledge of pharmacovigilance
A study conducted among pharmacists in Delhi revealed that 60% of the pharmacists lacked a good knowledge regarding pharmacovigilance and 72.30% of the pharmacists did not understand the meaning of ADR and the difference between an ADR and a side effect.14 In another study involving medical and dental practitioners in Karnataka, only 2.6% of the respondents reported to have received training with reference to ADR reporting and 46.4% of them reported that they had not reviewed even a single article regarding an ADR in a month. It was noted that more than 57.6% doctors had a pharmacovigilance knowledge level of <50%.15
Unawareness of reporting centres and unavailability of reporting forms
The study among the pharmacists also revealed that most of them were unaware of the reporting centres. Only 13.51% of the pharmacists reported that they had some set procedures of ADR reporting in their organization. Most pharmacists reported ADRs to Physicians, Manufacturing Industries, Department in-charge, Product Management Team, Chief Pharmacist and purchasing department of hospitals which further lowered the possibility of these reports to reach the National Monitoring Centres (NMCs) and Regional Monitoring Centres (RMCs). Only 2.70% of the pharmacists had the ADR reporting forms.14 Another observational, questionnaire-based study conducted in Hyderabad involving medical doctors working in different fields revealed 93.61% of the medical doctors agreed that ADR reporting forms were not available at their work place. 89.36% of the doctors accepted that they did not have knowledge about ADR reporting centres and 80.85% physicians agreed that they were not adequately trained in ADR reporting. 16
Problem in identifying possible ADRs
Another prevalent factor affecting under-reporting is that the healthcare professionals are not adequately trained in identifying ADRs. Clinicians were seen to face a challenge in identifying an ADR, the suspected drug and separating the relative contributions of drugs and disease processes to a patient’s chief complaint. Furthermore, regulatory-based passive surveillance systems are not set up to provide practical assistance in interpretation of possible ADRs, as a result of which many go unrecognised.17 It was also seen that having insufficient clinical knowledge among healthcare professionals made it difficult for them to decide whether a finding is an ADR or not. It was observed that among pharmacists, the ones working in the hospitals were more aware of pharmacovigilance, ADRs, expected therapeutic effects of drugs, possible side effects of prescribed drugs and pharmaco-economics than community pharmacists and medical representatives and hence were more involved in reporting ADRs than the medical representatives and community pharmacists. This may be due to the fact that not all community pharmacists and medical representatives have a pharmacy qualification as compared to hospital pharmacists. 14
Limitations of direct patient reporting
Some healthcare professionals feel that ADRs should be reported by physicians and the pharmacists.16 However, there are some physicians who support direct ADR reporting by the patients as well. Direct and spontaneous patient reporting offers added value for pharmacovigilance because it can speed up the acquisition of knowledge about ADRs and also because patients have a vested interest in reporting the adverse events affecting their lives.18 However, the consumers often have limited understanding of the drug safety system and do not have a clear idea of how to report these adverse events and where to report them. Many companies have started using social media as platforms to acquire information related to adverse events shared by consumers. However, social media has not caught on as a sanctioned source of ADR reporting as it is very difficult to interpret.19 Consumers write whatever they think and unlike reports from physicians, they often describe how the ADRs affect their lives.18, 19 What we get are mostly bits and pieces of an adverse event. That makes it extremely difficult to evaluate and understand whether it's a reportable event, let alone who to follow up with if it is.19 Another problem faced with direct reporting by patients is that most often patients are concerned more about the treatment of the ADRs than their reporting.17 This can be due to the fact that they are not aware of the importance of ADR reporting and how it can in turn affect their lives. Thus, it is important to impart this knowledge of pharmacovigilance in patients as well. This responsibility in turn lies with the physicians, nurses and pharmacists who interact with patients in person. They need to encourage patients to report any adverse effects they might be facing after using a drug and also direct them to the correct authority and facility where the patients can report these reactions. However, the data collected from the questionnaire based study involving pharmacists revealed that the interaction between pharmacists and patients were indeed quite low. 58% of the pharmacists informed the patients about the expected therapeutic effects of the drugs they would take while the rest did not. 48% of the pharmacists reported that they inform the patients about the likely side effects of their drug treatment. 45% of the pharmacists said that patients inform them about the discomfort and side effects experienced by them during or after a drug treatment while 37% pharmacists said patients do not interact with them about such effects. Pharmacists should extend their role from just dispensing to a responsible pharmacist who is willing to inform patients about the expected therapeutic effects, dosage regimen, directions for use and possible side effects of drugs.14
Other Factors
ADR reporting is voluntary in most countries. It was observed that some physicians do not report ADRs because of the lack of incentives or because they feel they should rather collect the data and publish themselves.20 Healthcare professionals have also admitted to have not reported adverse reactions due to the fear of legal liabilities, being labelled as careless and fear of admitting harm to patients.20 They have also cited that the ADR reporting forms are usually quite complicated which makes the process of reporting time consuming.16, 20 They also have an opinion that only safe drugs are available in the market and one report of adverse effect would not make any difference. 20
Discussion
Pharmacovigilance is still in a developing phase, especially in India, and there exists limited knowledge about this discipline. It was interesting to note in one of the studies conducted in Delhi that knowledge, skill and attitude of hospital pharmacists about pharmacovigilance and ADR reporting was the highest. This may be due to their pharmacy education and constant contact with healthcare professionals and patients in the hospital setting. In addition, all of them cleared the stringent selection procedures ensuring good knowledge base while entering the government sector.14 This shows the importance of increasing awareness of pharmacovigilance among all healthcare professionals and consumers as that would influence the attitude and practice of ADR reporting. Healthcare professionals should periodically be educated about adverse reactions and should be encouraged to report the same.4 Educational interventions can be targeted at multiple points in the profession like curriculum, internship training, seminars and Continuing Medical Education programmes.15 Students should be taught principles of drug safety and rational drug use in their undergraduate and postgraduate curriculum.4 The National Coordination Centre (NCC) of Indian Pharmacopoeia Commission (IPC) initiated a flagship programme called
"Skill Development Programme on Basics and Regulatory Aspects of Pharmacovigilance" in January 2017 at Ghaziabad, The programme was a part of the skill development programme in India under the government’s plan to produce and nurture qualified pharmacovigilance personnel for effective pharmacovigilance. It has till date trained 300 healthcare professionals including doctors and pharmacists to acquire requisite skills for furthering the Pharmacovigilance Programme of India (PvPI) in the country. More such initiates should be taken by the government.
Additionally, healthcare professionals should be encouraged to report any suspected adverse events they feel are important irrespective of proof of evidence or absolute certainty on whether it is related to the particular drug. Presence of trained surveillance personnel at hospitals to guide the clinicians would also help them in gaining more confidence in identifying ADRs and reporting them. Furthermore, healthcare professionals should be made aware of the various safety monitoring centres and trained in sending these reports to the appropriate authorities.
ADR reporting forms should be made readily available in hospitals and pharmacies and should be made relatively simple and easy to fill so that HCPs feel more inclined to report. Physicians, nurses, pharmacists should be trained on how to use these forms and should in turn be asked to guide consumers for direct patient reporting. Patients also need to be educated regarding the meaning of ADR, ADR reporting and its importance. Efforts should be made to make the reporting process by patients simple and straightforward, possibly by providing consumer online reporting forms or interactive tools. The Indian Pharmacopoeia Commission launched the adverse drug reaction reporting android application “ADR PvPI” in 2015 where healthcare professionals and consumers can instantly report adverse reactions due to a particular drug to PvPI from any part of India. Consumers and healthcare professionals should be made aware of such tools and applications whereby they can report any adverse reactions they want to.
Information regarding ADRs change on a daily basis and hence needs to be constantly updated. Most information in drug inserts and textbooks may be outdated and may not reflect the current state of information on ADRs. Hence ADR reporting guidelines should be made available in the form of booklets and posters at conspicuous locations in healthcare facilities and also on the internet to serve as a constant reminder.16 The new safety information obtained about a drug through this monitoring should be communicated to the health professionals, thus reducing further incidents of ADRs. Feedback from ADR monitoring centres about the causality and severity of ADRs reported by physicians would also encourage them to continue reporting.16 These measures would increase the faith of HCPs and consumers in the drug safety monitoring system and encourage them to participate more.
The reporting process should also be made anonymous which would reduce the fear of legal obligations. HCPs should be reminded that occurrence of adverse reactions are natural accompaniments of drug treatment due to their inherent properties.
They can be prevented through diligent and rational use of drugs. A physician thus cannot be held responsible for the occurrence of such reactions provided he has not been rash or negligent in using them. Therefore, no clinician should refrain from reporting on this basis.4 In most countries, doctors report adverse drug reactions to the authorities on purely voluntary basis. But in some countries they are required to do so legally. By making the process of ADR reporting mandatory, it should definitely increase the number of reports received. In March 2016, a notification was issued to pharmaceutical industries by Drug Controller General of India (DCGI), asking them to put in place a pharmacovigilance system and have a qualified person who would be responsible for the management of pharmacovigilance in the company. With this, pharmaceutical companies are now required to start pharmacovigilance activity which would in turn improve adverse drug reporting (ADR) to PvPI. However, entrusting pharmaceutical companies with the role of post marketing studies of safety and efficacy of their own drugs could have a conflict of interest as post marketing surveillance research can have huge financial implications. The possibility of withdrawing a drug from the market and the potential legal liability related to findings of serious side effects may lead to significant pressure on investigators, institutions and those working directly under contract with pharmaceutical sponsors. It is thus required to minimize conflicts of interest that can introduce bias in research design and reporting by researchers with ties to the industry. Monitoring and research that affect regulation of drugs should be free from industry influence and ought to have integrity. There can also be a conflict of interest when the agency that approves new drugs is also in charge of conducting post marketing studies on those same drugs, the results of which can lead to their withdrawal and could suggest a failure in the approval process. A fully independent agency or centre not involved in the drug approval processes and with no financial interest in drug development would thus be a better decision maker of safety and efficacy of post-marketed drugs. 21
The need to ensure that marketed drugs are safe and effective is of paramount importance and is also a moral obligation of government health and regulatory agencies. Regulators should take a proactive role in shaping safety and effectiveness surveillance and research, and engage in pre-emptive decision-making in order to prevent harm. This decision making is facilitated by the collection of the highest quality of evidence possible. Regulators also have the duty to be transparent with the public and warn people.22