Introduction
The skin is an organ made up of tissues that are structurally joined together to perform various activities.1 The skin of an adult human body has an area of around 2m and receives nearly one-third of the total blood circulating in the body.2 Percutaneous absorption of drug by skin occurs primarily through stratum corneum. Stratum corneum consists of dead epidermal keratinized cells with a thickness of 10m and serves as a barrier for drug permeation. Hence it is difficult to transport the drug molecules across the skin.3 The rate and extend of drug absorption via the skin depends upon the physiology of the skin, physiochemical characteristics of the drugs and the delivery system. In terms of the ease of application through self- administration, the topical route provides a broader and diverse surface and offers an alternative to oral drug delivery as well as hypodermic injection. Topical dosage forms like patches, ointment, creams have major drawbacks like skin irritation, sweat ducts obstruction and poor physical appearance, semi-solid preparations have poor patient compliance and do not provide tenacious contact with the skin surface and can be cleaned off quickly by the clothes of the patient.4 Therefore, a dosage form needs to be developed that allows less frequent dosing by establishing less contact with the skin for an extended period of time, thus improving patient compliance. Transemulgel is a subcutaneous Novel approach that can be used as an alternative to conventional topical and transdermal formulations.5 It is defined as a non-solid dosage form producing an in situ film after application to the skin or any other surface of the body. Such system includes the drug and film forming excipients in a vehicle that, when contact with the skin, creates a film of excipients on solvent evaporation along with the drug. The film developed is a residual film of liquid which is easily absorbed into the subcutaneous layer.6
Mechanism of film formation and permeation
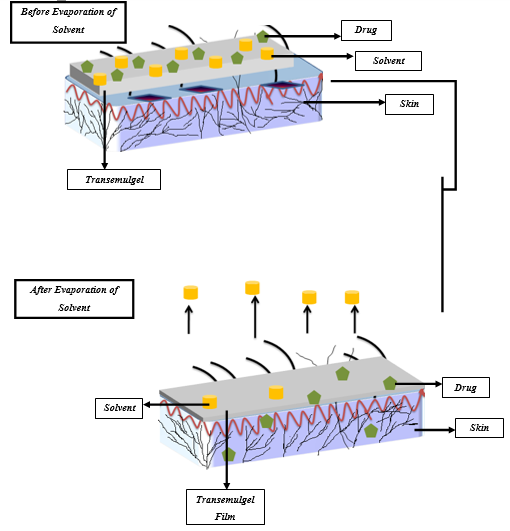
Transemulgel are applied directly to the skin, which forms in situ, thin and transparent film after solvent evaporation (Figure 1).7 Once the formulation has been applied to the skin, the composition of the transemulgel changes considerably due to the loss of the vehicle’s volatile substance resulting in the creation of persistent film on the skin surface. In this system the concentration of the drug increases, reaching the level of saturation and with the potential of reaching a level of supersaturation on the surface of the skin.8
Supersaturation results in increased drug flux through the skin by increasing the thermodynamic activity of formulation without affecting the skin barrier, thus eliminating the side effects and soreness.9
The theory of supersaturation can be explained by Fick’s modified form of diffusion law
here,
From the above equation it is clear that the drug permeation rate through the skin is proportional to the drug concentration. It is possible even when the whole drug in the vehicle is dissolved. Equation (ii) defines the modified form of Fick’s diffusion law:
here,
According to equation (ii), the drug flux is directly proportional to the saturation-related thermodynamic behavior of the system. So, an increase in supersaturation increases the instability of thermodynamic.10
Transemulgel develops supersaturated system immediately after application to the skin and overcomes the instability issue. This thus, increases the permeation of the medication across skin as compared to other types of transdermal delivery.11
Factors affecting the composition of Transemulgel
Adequate Medicines
This must specify specific criteria for a transdermal application that are self- reliant of the dosage form. Since the skin is a very effective defensive barrier for the body, not only against physical or microbial noxes but also against drugs, only strong drugs with a regular dose of less than 10mg are suitable for this route of application. To provide adequate mobility in the skin structures, the size of the molecule must be small i.e. molecular weight below 500Da. The partition coefficient (log P) of the drug must be in between 1 and 3, since the drug has to pass through both lipophilic and hydrophilic areas of the skin on its route into the systemic circulation. For this purpose molecules with a pH value between 5 and 9 are required in aqueous solution for transdermal application.12 Other criteria that is important for the transdermal delivery of drugs are- A minimal number of hydrogen bonding group (<2) and a low melting point (less than 200oC). Transemulgel mainly desirable for the drugs that have following characteristics-
High Potency- results in low daily doses required for the medication.
High Permeability to the skin- promotes high thin reservoir efficiency for polymer
High Solvent Solubility- enables high drug loading and a high gradient formed between formulation and skin.13
An effective delivery system is typically beneficial for all medications, ensuring a high use of the drug reservoir across the entire wearing time. If this cannot be accomplished, the transemulgel could not be accepted to sophisticated drugs because of the significant amount of drug loss.14 With regard to the novelty of transemulgels, a comprehensive and individual evaluation is still necessary for each new drug candidate before a better understanding of the characteristics of transemulgel in general has been obtained.12
Adequate Excipients
Polymer
The polymer is needed to create thin film at skin surface temperature (28oC- 30oC) and should have some intrinsic versatility and sensitivity to the skin to prevent the use of excessive amount of plasticizer. It must be soluble in a skin friendly and highly volatile solvent.15 In addition, as a film former, intense gelling agents should be avoided as they prohibit spreading from applying the formulation. Considering numerous requirements the polymer screening tests have shown that most of the polymers tested can be incorporated into a transemulgel composition with adequate and appropriate macroscopic properties.16
Although polymers are films developed with sufficient macroscopic properties, but permeation analysis have shown that certain polymers are superior to others in terms of drug delivery.17 The study revealed that not only the polymers immobilize the drugs in a skin matrix, but they would also have an enhancing and retarding effect on the permeation of drug. This effect benefit in two ways, the one is form complex interaction between the polymer formation and the skin and the other form interactions between the polymer and the drug.15 Depending on the physico-chemical properties of the two molecules, such as charge or lipophilicity, the intensity of the latter is different for each drug polymer combination. For the composition of a transemulgel of a new drug candidate this should be remembered.18
Solvent
The solvent is also a very essential compound in the transemulgel, but due to its rapid evaporation it is not part of the original film on the skin. The solvent should offer enough solubility for both the polymer and the drug.19 Only a high solvent solubilizing capacity for the drug allows significant variations in the drug charge to alter drug distribution to the skin. The solvent can also have a direct effect on the drug flux.20 Depending on the nature of the solvent and its permeation enhancing properties, even with its short processing time with the skin, it can facilitate drug transfer to different levels. In addition to its solubilizing properties, an effective solvent for transemulgel is needed for the polymer and the drug to be extremely volatile in order to provide quick drying time and thus a good patient compliance.19 After application it should spread well on the skin and along with the polymer to produce a smooth film by a uniform thickness on the targeted site.20 Water cannot be considered an appropriate solvent for preparation of transemulgel polymeric composition. Solvents like ethanol, isopropanol, or ethyl acetate with high volatility and strong stretching should be preferred.21
Plasticizer
In polymeric solutions the main aim of plasticizer is to enable the developing of films and to increase the intensity of the resulting film. With context to the film former, the plasticizer must be accurately selected. Because the performance of a plasticizer is polymer dependent, no specific rule can be applied as to what concentration of plasticizer is required to manufacture films with the required characteristics. The determination of the correct plasticizer content by individual is unavoidable.21 Insufficient excipient concentrations tend to fragile films with poor adhesion to the skin. On the other hand, an excessive amount of plasticizer contributes to smooth, but sticky films. Both situations are inappropriate for transemulgel to deliver credible drugs and a good patient compliance. Usually the plasticizer should have low permeability towards the skin to avoid leakage from the developed film.22 A significant leakage not only raise safety issues, but it would also leads to a reduction of the film properties. When a plasticizer loss occurs the transemulgel film gets brittle and loses some of its adhesive properties.23
Further Excipients
In addition to the basic substances of a transemulgel polymeric solution (polymer, solvent and plasticizer), it could be suitable to include additional excipients in the formulation.24 The compatibility of the compounds with all other substances is a prerequisite for the application of further excipients. It should be noted that any change in the composition of the transemulgel could have a negative effect on the macroscopic properties of the prepared transemulgel such as stability, skin adhesion or stickiness of the outer layer of the film.25 Hence, it is recommended to re-examine the macroscopic properties of the formulated transemulgel after some structure modification.
Evaluation Parameters of Transemulgel
Phase Transition Time: The time transemulgel takes for the gel to be transferred to the film is the transitional time. One gm of gel was dropped on a petri dish that was uniformly spread over it and placed on a 37oC hot plate and the time required till gel converts were measured into film.26
Film Weight: One gm of the gel was dropped in a petri dish and had been kept for drying. The resulting film was then weighed on an electronic balance after drying.26
Rheological studies: The Brookfield Viscometer- “LVDV II” had been used to evaluate the rheology of the formulated transemulgel. To determine the viscosity of transemulgel, gels were placed under the viscometer using a S 64 spindle. The viscosity was measured at various RPM like- 10, 20, 50, 100 and the resulting viscosity and torque was noted.27 .
Spreadability Studies: Specific amount of the formulation was kept between two glass plate and the glass plate on the top was slide gently onto the bottom glass slide to evaluate the spreadability of the formulation based on the drift and sliding characteristics of the gel. At this point a ground glass slide had fastened and to this slide about 2gm of gel was kept under examination. The gel was then squeezed and supplied with the connection between these two slides having the proportion of fixed ground slide. To remove air and create a uniform film of the gel between the slides, 1 kg weight was put on top of the two slides for 5 min. the residual gels from the edges was removed off. Then, the top plate was prone to 80gm pressure by using the string attached to the loop and the time (in sec) taken by the top slide to complete a distance of 7.5 cm was noted.28 Better spreadability had been demonstrated by a shorter time. The spreadability was evaluated by following formula:
here,
S= spreadability,29
Conclusion
Transemulgel indicates to be an efficient treatment for transdermal drug delivery. This also tends to stay adhere to the effected portion for a prolonged period of time without being rubbed off. It offers continuous effect and better relief than traditional gels, and does not require re-application. The concept of transemulgel can change the framework for treatment of various diseases, like arthritis. There is a lot of work to be done in this field. This system will be used widely in coming years, because of its various properties like greater patient compliance, less irritation, spreadability, adhesiveness, etc. Not only this, they can become an option for hydrophobic drugs to be loaded into water soluble gel bases.