Introduction
What are coronaviruses?
Coronaviruses (CoVs) are a large family of viruses that cause illness ranging from the common cold to more severe diseases such as Middle East Respiratory Syndrome (MERSCoV) and Severe Acute Respiratory Syndrome (SARS-CoV). A novel coronavirus (nCoV) is a new strain that has not been previously identified in humans.1
Coronaviruses are large, enveloped, positive-stranded RNA viruses. They have the largest genome among all RNA viruses. The genome is packed inside a helical capsid formed by the nucleocapsid protein and further surrounded by an envelope. Associated with the viral envelope are at least three structural proteins: the membrane protein and the envelope protein are involved in virus assembly, whereas the spike protein mediates virus entry into host cells. Among the structural proteins, the spike forms large protrusions from the virus surface, giving coronaviruses the appearance of having crowns (hence their name; corona in Latin means crown). In addition to mediating virus entry, the spike is a critical determinant of viral host range and tissue tropism and a major inducer of host immune responses. (Li, 2016) Birds and mammlas commonly affected by coronaviruses subsequently causing diversified fatal diseases. Generally respiratory, gastrointestinal and central nervous system will get affected by coronaviruses in humans and other animals which are not only life threatening to human but also causing economic loss. (Li, 2016) Coronaviruses are efficient enough for adjusting to varied environments through mutation and recombination with proficiency and can affect new hosts and tissues with ease.
Rarely, certain coronaviruses can also affect certain animal species and can generate new strains which can cross over to human hosts and finally get transmitted between humans. In past, humans had never been exposed to such kind of viruses, therefore even vaccines are not available not even humans have natural immunity for these. This is the reason their mutations can swiftly lead to outbreak of new kind of disease and eventually into pandemics. SARS and MERS are the examples of such outbreaks.
Coronaviruses derived the specific termology from the characteristic crown-like viral particles (virions) that present on their surface like a dot.1, 2, 3, 4 With the recent detection of the 2019 novel coronavirus (COVID-19), there are now a total of 7 coronaviruses known to infect humans.
Human coronavirus 229E (HCoV-229E
Human coronavirus OC43 (HCoV-OC43
Human coronavirus NL63 (HCoV-NL63
Human coronavirus HKU1
Severe acute respiratory syndrome-related coronavirus (SARS-CoV
Middle East respiratory syndrome-related coronavirus (MERS-CoV
Novel coronavirus (COVID-19, also known informally as Wuhan coronavirus)2
Novel coronavirus (COVID-19, also known informally as Wuhan coronavirus 2
Prior to the global outbreak of SARS-CoV in 2003, HCoV-229E and HCoV-OC43 were the only coronaviruses known to infect humans. Following the SARS outbreak, 5 additional coronaviruses have been discovered in humans, most recently the novel coronavirus COVID-19, believed to have originated in Wuhan, Hubei Province, China. SARS-CoV and MERSCoV are particularly pathogenic in humans and are associated with high mortality. The present review summarizes the epidemiology, pathogenesis, and clinical characteristics of COVID-19 with the current treatment procedures and future scientific advancements to combat the epidemic novel coronavirus.
Origin and transmission of SARS-CoV-2
The SARS-CoV-2 is a β-coronavirus, which is enveloped non-segmented positive-sense RNA virus (subgenus sarbecovirus, Orthocoronavirinae subfamily) 3Coronaviruses (CoV) are divided into four genera, including α−/ β−/γ−/δ-CoV. α- and β-CoV are able to infect mammals, while γ- and δ-CoV tend to infect birds. Previously, six CoVs have been identified as human-susceptible virus, among which α-CoVs HCoV-229E and HCoV-NL63, and β-CoVs HCoV-HKU1 and HCoV-OC43 with low pathogenicity, cause mild respiratory symptoms similar to a common cold, respectively. The other two known β-CoVs, SARS-CoV and MERS-CoV lead to severe and potentially fatal respiratory tract infections 5 was found that the genome sequence of SARS-CoV-2 is 96.2% identical to a bat CoV RaTG13, whereas it shares 79.5% identity to SARS-CoV. Based on virus genome sequencing results and evolutionary analysis, bat has been suspected as natural host of virus origin, and SARSCoV-2 might be transmitted from bats via unknown intermediate hosts to infect humans. It is clear now that SARS-CoV-2 could use angiotensin-converting enzyme 2 (ACE2), the same receptor as SARS-CoV 6 to infect humans.
How is SARS-CoV-2 coronavirus transmitted? 7
a. The transmission of SARS-CoV-2 occurs by the following mechanisms:
Commonly spread through close contacts between two persons (about less than 6 feet/1 8 metres
b. Other prevalent reason for person-to-person spread is primarily via respiratory droplets produced when an infected person coughs or sneezes, which is similar to the spread of influenza and other respiratory pathogens.
c. Respiratory droplets further can go inside the mouth, noses or eyes of people who came in contact with the infected person or in serious transmission can be inhaled into the lungs.
d. Even by touching the surface or any object where the virus reside can be the source of infection of COVID-19 and get transmitted to the person by touching and further reaches to their facial part. But this is not the primary way of virus transmission (Centres for Disease Control and Prevention, 2020). Infact it is evident that coronaviruses can remain live on inanimate surfaces for several hours or even days. (Kampf G, 2020).
e. In cases of most respiratory viruses, the most symptomatic one is supposed to be most contagious. But COVID-19 reports have suggested even the spread occurs from an asymptomatic infected person to any close contact. (Centres for Disease Control and Prevention, 2020) (Rothe, 2020). 8 Ongoing researches suggest that asymptomatic (or pre-symptomatic) patients not only transmits the COVID-19 infection but also the expansion of the disease is really fast (Ruiyun Li, 2020). 9
f. With 10- 14 days of long incubation period and even after the remission of symptoms patients can remain contagious for one to two weeks. According to research done by Wölfel et al, symptoms only remains for the first week but even though viral RNA can be detectable in throat swabs for upto second week. Stool and sputum samples remained RNA positive for longer periods. (Roman Wölfel, 2020) 10
g. Information regarding COVID-19 affect during pregnancy is minutest and intrauterine or perinatal transmission has not been identified till now. There are reports of 18 pregnant women with COVID-19 infection with neonates have no presence of the virus. However, two neonatal cases of infection have been documented with COVID-19 positive mother. In one case, the diagnosis was made at day 17 of life after close contact with the infant's mother and a maternity matron who were both infected with the virus. The other case was diagnosed 36 hours after birth; the source and time of transmission in that case were unclear. (McIntosh, Coronavirus disease 2019 (COVID-19) - Special situation: Pregnant women, 2020). Several developed countries suggested about pregnant women to remain isolated as preventive measures because there is increased risk of infection. 11, 12
h. Althogh sparse data suggest that in coronovirus infected mothers, the virus has not been detected in their breast milk. As we all know breast milk provides immunity and protection against many diseases to the neonates but there is no assurance about mothers with COVID-19 can transmit the virus via breast milk.
i. The CDC has no specific guidelines for continuing the breastfeeding during infection of mothers with any type of coronaviruses including with SARS-CoV or Middle Eastern Respiratory Syndrome (MERS-CoV) or even COVID19. CDC recommends proper precautions should be taken during breastfeeding to avoid any kind of transmission of infection to the neonate. WHO highlights the less chances of transmission of respiratory viruses through breast milk, and currently suggest that mothers with COVID-19 can breastfeed. (Academy of Breastfeeding Medicine, 2020)
Epidemiology − reservoirs and transmission
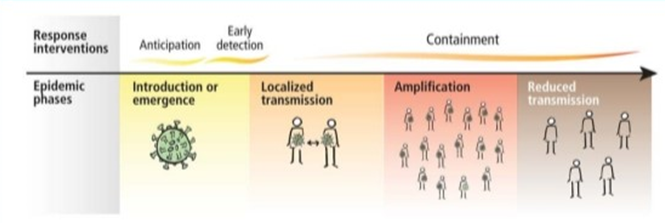
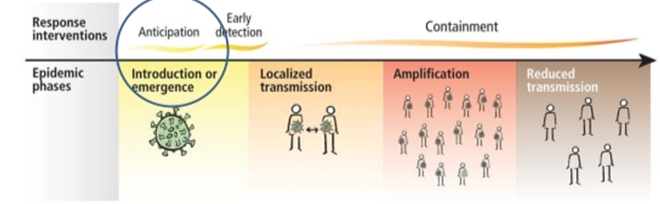
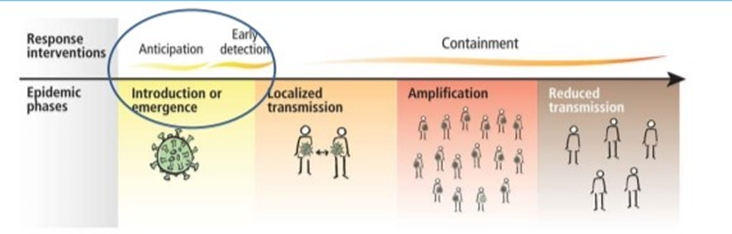
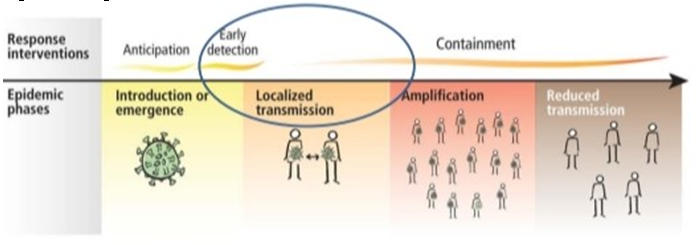
The current epidemic of acute respiratory tract infection of unknown origin spread out initially in Wuhan, China, since early December 2019, which later suggest its relation to a seafood market. Further research concluded that bat can be the potential reservoir of SARS-CoV-2. 13, 14 Till now, the origin of SARS-CoV-2 from the seafood is not established evidentially. Preferably, the fact that bats are the natural reservoir of a wide variety of CoVs, including SARS-CoV-like and MERS CoV-like viruses is far better accepted. 15, 16 Upon virus genome sequencing, the COVID-19 was analyzed throughout the genome to Bat CoV RaTG13 and showed 96.2% overall genome sequence identity5 suggesting that bat CoV and human SARS-CoV-2 might share the same ancestor, although bats are not available for sale in this seafood market 17 Besides, protein sequences alignment and phylogenetic analysis 18 showed that similar residues of receptor were observed in many species, which provided more possibility of alternative intermediate hosts, such as turtles, pangolin and snacks. Human-to-human transmission of COVID-19 mainly occurs due to close contact with the patients or incubation carriers. A report declares that 19 31.3% of patients were the ones who recently visited Wuhan and 72.3% of patients contacting with people from Wuhan among affected nonresidents of Wuhan. National Health Commission of China declares that COVID-19 transmission occurred in 3.8% healthcare workers. In contrast, the transmission of SARS-CoV and MERS-CoV was reported to occur primarily through nosocomial transmission. Infections of healthcare workers in 33–42% of SARS cases and transmission between patients (62–79%) was the most common route of infection in MERS-CoV cases. 20, 21 Direct contact with intermediate host animals or consumption of wild animals was suspected to be the main route of SARS-CoV-2 transmission. However, the source(s) and transmission routine(s) of SARS-CoV-2 remain elusive.
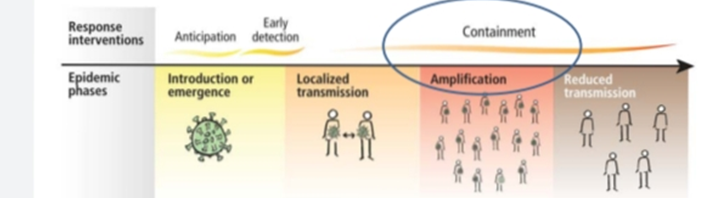
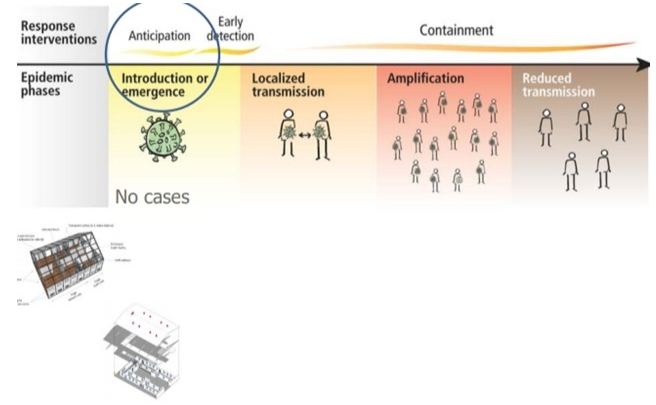
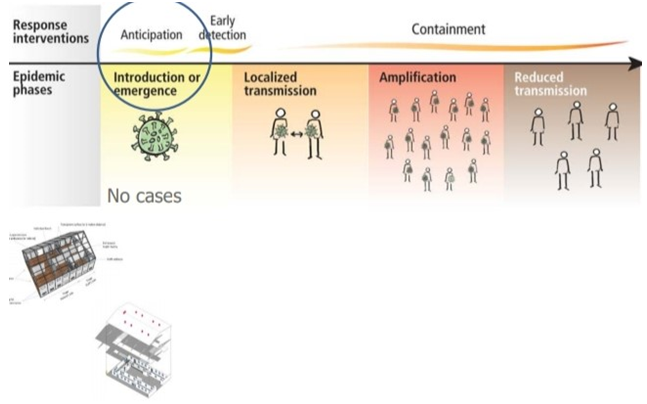
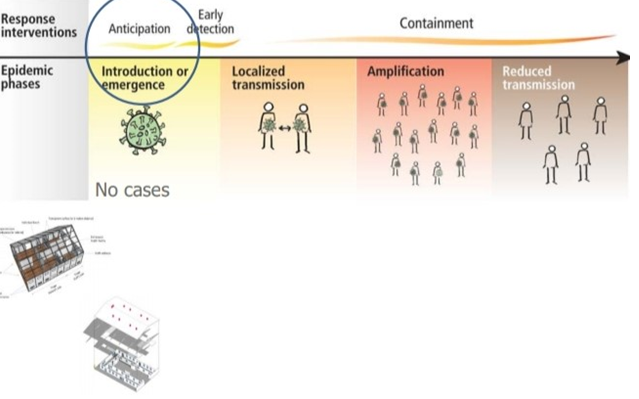
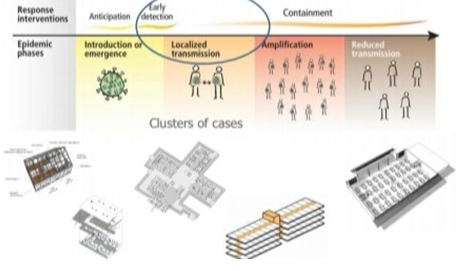
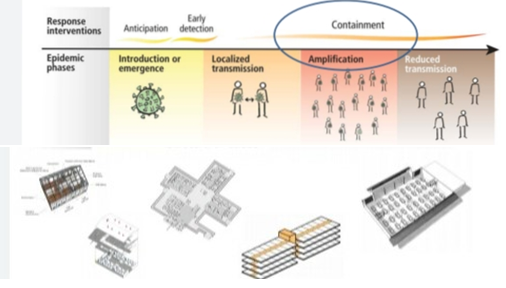
Four transmission scenarios are observed.
Four transmission scenarios are observed.
Countries with no cases (no cases)
Countries with one or more cases imported or locally acquired (sporadic cases)
Four transmission scenarios are observed.
Countries with no cases (no cases)
Countries with one or more cases imported or locally acquired (sporadic cases)
Countries experiencing clusters of casas in time geographic location or common exposure (clusters of cases)
Four transmission scenarios are observed.
Countries with no cases (no cases)
Countries with one or more cases imported or locally acquired (sporadic cases)
Countries experiencing clusters of casas in time geographic location or common exposure (clusters of cases)
Countries experiencing larger outbreaks of local transmission (community transmission).
• Set up screening and triage
• Set up COVID 19 designated wards in health facilities.
• COVID-19 designated treatment area
• Repurpose existing buildings
• Community facilities.
Clinical symptoms
A recent study led by Prof. Nan-Shan Zhong’s team, by sampling 1099 laboratory-confirmed cases, found that the common clinical manifestations included.
fever (88.7%), sputum production (33.4%),cough (67.8%)shortness of breath, (18.6%), fatigue(38.1%), sore throat(13.6%), headached 17 In addition, a part of patients manifested gastrointestinal symptoms, with diarrhoea (3.8%) and vomiting (5.0%). The clinical manifestations were in consistence with the previous data of 41, 99, and 138 patients analysis in Hubei province. 22, 23, 24 Fever and cough were the dominant symptoms whereas upper respiratory symptoms and gastrointestinal symptoms were rare, suggesting the differences in viral tropism as compared with SARS-CoV. 25 MERSCoV. 26 and influenza. 27 The elderly and those with underlying disorders (i.e., hypertension, chronic obstructive pulmonary disease, diabetes, cardiovascular disease), developed rapidly into acute respiratory distress syndrome, septic shock, metabolic acidosis hard to correct and coagulation dysfunction, even leading to the death. 28 In laboratory examination results, most patients had normal or decreased white blood cell counts, and lymphocytopenia 19, 29 But in the severe patients, the neutrophil count, D-dimer, blood urea, and creatinine levels were higher significantly, and the lymphocyte counts continued to decrease. Additionally, inflammatory factors (interleukin (IL)-6, IL-10, tumournecrosis factor-α (TNF-α) increase, indicating the immune status of patients. The data showed that ICU patients had higher plasma levels of IL-2, IL-7, IL-10, granulocyte colony-stimulating factor (GCSF), 10 kD interferongamma-induced protein (IP-10), monocyte chemoattractant protein-1 (MCP-1), macrophage inflammatory protein 1-α (MIP-1α), and TNF-α. 28 Moreover, the CT imaging showed that computed tomography on the chest was ground-glass opacity (56.4%) and bilateral patchy shadowing (51.8%), 19 sometimes with a rounded morphology and a peripheral lung distribution, analyzed from the patients in the Fifth Affiliated Hospital, Sun Yat-Sen University. 30 Clinicians have been aware that, a part of confirmed patients appeared the normal CT image presentations. The diagnostic sensitivity of radiologic is limited, so it is necessary to verify with clinical symptoms and virus RNA detections.
Treatment of COVID-19
Current therapies Given the lack of effective antiviral therapy against COVID-19, current treatments mainly focused on symptomatic and respiratory support according to the Diagnosis and Treatment of Pneumonia Caused by COVID-19. issued by National Health Commission of the People’s Republic of China. 31 Nearly all patients accepted oxygen therapy, and WHO recommended extracorporeal membrane oxygenation (ECMO) to patients with refractory hypoxemia. 32 Rescue treatment with convalescent plasma and immunoglobulin G 33 are delivered to some critical cases according to their conditions.Antiviral treatments Based on the experience of fighting the epidemic SARSCoV and MERS-CoV previously, we may learn some lessons for some treatment strategies against coronavirus. 34 Antiviral drugs and systemic corticosteroid treatment commonly used in clinical practice previously, including neuraminidase inhibitors (oseltamivir, peramivir, zanamivir, etc), ganciclovir, acyclovir, andribavirin,as well as methylprednisolone. 22, 3 for influenza virus, are invalid for COVID-19 and not recommendedRemdesivir (GS-5734) is a 1′-cyano-substituted adenosine nucleotide analog prodrug and shows broadspectrum antiviral activity against several RNA viruses. Based on the data collected from in vitro cell line and mouse model, remdesivir could interfere with the NSP12 polymerase even in the setting of intact Exon proofreading activity. 35 Remdesivir has been reported to treat the first US case of COVID-19 successfully. 36 Chloroquine is a repurposed drug with great potential to treat COVID-19. Chloroquine has been used to treat malaria for many years. 36 with a mechanism that is not well understood against some viral infections. Several possible mechanisms are investigated: Chloroquine can inhibit pH- The other antiviral drugs include nitazoxanide, favipiravir, nafamostat (See Table 1 for details).
Table 1
Common and potent antiviral drugs
dependent steps of the replication of several viruses, 37 with a potent effect on SARS-CoV infection and spread. 4 Moreover, chloroquine has immunomodulatory effects, suppressing the production/release of TNF-α and IL-6. It also works as a novel class of autophagy inhibitor,38 which may interfere with viral infection and replication. Several studies have found that chloroquine interfered with the glycosylation of cellular receptors of SARS-CoV 39 and functioned at both entry and at post-entry stages of the COVID-19 infection in Vero E6 cells. 40 A combination of remdesivir and chloroquine was proven to effectively inhibit the recently emerged SARS-CoV-2 in vitro. Scientists previously confirmed that the protease inhibitors lopinavir and ritonavir, used to treat infection with human immunodeficiency virus (HIV), 41 could improve the outcome of MERS-CoV42 and SARSCoV43 patients. It has reported that β-coronavirus viral loads of a COVID-19 patient in Korea significantly decreased after lopinavir/ritonavir (Kaletra®, AbbVie, North Chicago, IL, USA) treatment. 44 Additionally, clinicians combined Chinese and Western medicine treatment including lopinavir/ritonavir (Kaletra), arbidol, and Shufeng Jiedu Capsule (SFJDC, a traditional Chinese medicine) and gained significant improvement in pneumonia associated symptoms in Shanghai Public Health Clinical Center, China. 45
Originating from reservoir of bats and unknown intermediate hosts, SARS-CoV-2 binds to ACE2 with high affinity as a virus receptor to infect humans. Secondly, the susceptible population involves the elderly and people with certain underlying medical conditions, which requires more attention and care. Thirdly, so far, the supporting treatments, combined with potent antiviral drugs, such as remdesivir, chloroquine, or lopinavir/ritonavir, have been conducted with definite effect on treat COVID-19 patients, while solid data from more clinical trials are needed. However, questions remain vague and more studies are urgent to explore the transmission and pathogenicity mechanism of the emerging coronavirus. To make clear the evolutionary path from the original host to cross-species transmission so as to potentially limit the transmission to animals or humans. In addition, to uncover the mystery of the molecular mechanism of viral entry and replication, which provides the basis of future research on developing targeted antiviral drugs and vaccines? Given more than 80% of patients are confirmed in Hubei province, the hospitals and medical workers in Hubei are facing and bearing enormous pressure and severe challenge, including a high risk of infection and
adequate protection, as well as overwork, frustration and exhaustion 61Chinese Government and authorities have launched psychological intervention, and we sincerely hope that Chinese people and other countries overcome the epidemic as fast as possible.
Convalescent plasma therapy
For COVID-19 patients with rapid disease progression, severe and critical illness, convalescent plasma therapy (CPT) can be tried (National Health Commission of the People’s Republic of China, 2020). CPT utilises a certain titre of virus-specific antibodies in the plasma of the convalescent individual to enable the patient receiving the infusion to obtain passive immunity and remove pathogens from the blood circulation. This method has been successfully used in the treatment of SARS and H1N1 influenza, and is an effective treatment (Chen L, 2020). 46
The use of CPT treatment can follow the following principles (National Health Commission of the People’s Republic of China, 2020)
In principle, the course of disease does not exceed three weeks. Also, the patient should have a positive viral nucleic acid test or viraemia certified by clinical experts.
Patients with severe disease with rapid disease progression, or critically ill early stage patients, or patients comprehensively evaluated by clinical experts as requiring plasma therapy. The infusion dose is determined according to the clinical situation and the weight of the patient, usually the infusion dose is 200500 ml (4-5 ml/kg).
Before, during, and after the infusion, detailed records and clinical observation should be made to assess the adverse effects of plasma infusion. The main types of adverse transfusion reactions include transfusion-related circulation overload, transfusion-related acute lung injury, transfusion-related dyspnoea, allergic reactions, transfusion-associated hypotension reactions, non-haemolytic febrile reactions, acute haemolytic transfusion reactions, and delayed haemolytic transfusion reaction, infectious transfusion reaction, other/unknown, etc.
Advances in vaccines development for the treatment of COVID-19
Since the vaccine development process involves procedures such as virus strain isolation and selection, in vitro experiments, animal experiments, clinical trials, and administrative approvals, it takes a long time. At present, some recognition sites for SARS-CoV-2 have been found and can be used for vaccine development (Ahmed SF, 2020) (Ramaiah A, 2020).63-64
The Ministry of Science and Technology of the People’s Republic of China has organised national key units to carry out joint research, and arranged five technical routes in parallel, including inactivated vaccines, recombinant genetically engineered vaccines, adenovirus vector vaccines, nucleic acid vaccines (mRNA vaccine and DNA vaccine), and vaccines made from attenuated influenza viral vaccine vectors.
Progress of clinical trials for the treatment of COVID-19
At present, clinical research projects on new coronavirus pneumonia drugs are ongoing. As of 9 am on 8 March 2020, a total of 436 clinical trials were retrieved from the Chinese Clinical Trials Registry, and a total of 181 clinical trials involving drug treatment were screened out, of which 107 were randomised controlled trials, four were real-world studies, and 70 were non-randomised controlled trials.Of the 181 studies, 176 were initiated by Chinese research institutions, mainly distributed in Hubei (43), Shanghai (25), Beijing (20), Zhejiang (20) and Guangdong (19). The remaining five studies were initiated by other countries.
The drugs involved in clinical trials mainly include traditional Chinese medicine (TCM) interventions (64 items), antiviral drugs (40 items), immunotherapy drugs (28 items, such as Interferon, Thymosin, Immunoglobulin, PD1 inhibitors, etc.), anti-malaria drugs (21 items, such as chloroquine, hydroxychloroquine, chloroquine phosphate), glucocorticoids (6 items), and other drugs (22 items, such as vitamin C, vitamin D, polymyocyte injection, zinc sulphate, acetylcysteine, etc.).
The most clinical trials of antiviral medicines are anti-HIV medicines (14 items, such as lopinavir/ritonavir,darunavir/cobistastat, azivudine), followed by anti-influenza viruses medicines (13, such as umifenovir, fapilavir), and five clinical trials of remdesivir, which are considered to have potential efficacy against COVID-19.
Prevention
To help control further spread of the virus, people who are suspected or confirmed to have the disease should be isolated from other patients and treated by health workers using strict infection control precautions.
People who have had social contact with symptomatic individuals with confirmed COVID19 should be followed up as a contact through the local healthcare teams
WHO’s standard recommendations for the general public to reduce exposure to and transmission of this and other respiratory illnesses are as follows, which include hand and respiratory hygiene, and safe food practices.65-66
Frequently clean hands by using alcohol-based hand rub or soap and water
When coughing and sneezing cover the mouth and nose with a flexed elbow or tissue – throw the tissue away immediately and wash hands.
Avoid close contact with anyone who has fever and cough
If you have fever, cough and difficulty breathing seek medical care early and share previous travel history with your healthcare provider.
When visiting live markets in areas currently experiencing cases of novel coronavirus, avoid direct unprotected contact with live animals and surfaces in contact with animals.
The consumption of raw or undercooked animal products should be avoided. Raw meat, milk or animal organs should be handled with care, to avoid crosscontamination with uncooked foods, as per good food safety practices. (World Health Organization, 2020).